
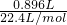 =0.04mol��
=0.04mol��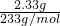 =0.01mol�����ɵij���BaCO3���ʵ���=
=0.01mol�����ɵij���BaCO3���ʵ���= =0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����
=0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����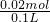 =0.2mol/L��C��SO42-��=
=0.2mol/L��C��SO42-��= =0.1mol/L��C��NH4+��=
=0.1mol/L��C��NH4+��= =0.4mol/L��������Һ�е���غ�õ���C��K+��+C��NH4+��=2C��SO42-��+2C��CO32-����C��K+��=0.2mol/L������Һ�к��������ӣ���C��K+����0.2mol/L��
=0.4mol/L��������Һ�е���غ�õ���C��K+��+C��NH4+��=2C��SO42-��+2C��CO32-����C��K+��=0.2mol/L������Һ�к��������ӣ���C��K+����0.2mol/L��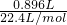 =0.04mol��
=0.04mol��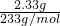 =0.01mol�����ɵij���BaCO3���ʵ���=
=0.01mol�����ɵij���BaCO3���ʵ���= =0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����
=0.02mol���������ӹ����֪��Һ��һ������Mg2+��K+��Cl-����ȷ����

 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ��������Ba2+��NH4+���ܴ��� | B��CO32-һ�������� | C��Na+һ������ | D��һ��������Cl- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com