
 ijѧ����ͼ�õ�ⷨ���ݵ缫���������ʵ���������֤�����ӵ�����ֵ����ʵ�鷽����Ҫ��Ϊ��
ijѧ����ͼ�õ�ⷨ���ݵ缫���������ʵ���������֤�����ӵ�����ֵ����ʵ�鷽����Ҫ��Ϊ��| m |
| 64 |
| 64 |
| m |
| It |
| 2��1.6��10-19 |
| 64 |
| m |
| It |
| 2��1.6��10-19 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Mg2+��Fe2+��SO42- |
| B��Ba2+��NH4+��Br- |
| C��Al3+��HCO3-��Cl- |
| D��Na+��SiO32-��ClO- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| A��ԭ�Ӱ뾶��W��Z��Y��X��M |
| B��������Y2M4��M2Z2��ֻ���м��Լ� |
| C������������Ӧˮ��������ԣ�Y��X |
| D����X��Y��Z��M����Ԫ���γɵĻ�����һ���������Ӽ������й��ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��6.89���ڵ�KHSO4�к���0.1NA�������� |
| B��46 g NO2��N2O4��������к���ԭ������Ϊ3NA |
| C����2H��18O����ɵ�ˮ11g������������������Ϊ4NA |
| D����״���£�2.24L Cl2ͨ������H2O��NaOH��Һ��ת�Ƶĵ�������Ϊ0.1NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��25�棬1.01��105Pa��64g SO2�к��еķ�����Ϊ2NA | ||
| B�����³�ѹ�£�11.2L Cl2���еķ�����Ϊ0.5NA | ||
| C����״���£�11.2L N2��CO�Ļ�����������Ϊ28g | ||
D��1mol N
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
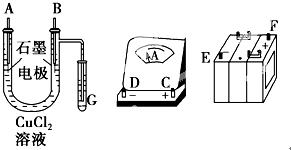
 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com