
øł¾ŻŅŖĒ󊓳öĻĀĮŠ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½
(1)Ņ»¶ØĮæĒāĘųÓėĀČĘų·“Ӧɜ³ÉĀČ»ÆĒāĘųĢå,µ±Éś³É1molĒāĀČ¼üŹ±·Å³ö91.5kJµÄČČĮæ________________________________________________________.
(2)ij»Æѧ·“Ó¦µÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾,øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ(·“Ó¦ČČÓĆabc±ķŹ¾)
_____________________________________________.
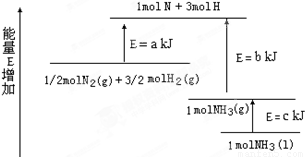
(3)ij·“Ó¦µÄĘ½ŗā³£Źż Čē¹ūÓŠ1molN2 ĶźČ«·“Ó¦,ŅŖĪüŹÕČČĮæ68kJ.Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½______________________________________________.
Čē¹ūÓŠ1molN2 ĶźČ«·“Ó¦,ŅŖĪüŹÕČČĮæ68kJ.Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½______________________________________________.
(4)ŹµŃéÖŠ²»ÄÜÖ±½Ó²ā³öŹÆÄ«ŗĶĒāĘųÉś³É¼×Ķé·“Ó¦µÄ·“Ó¦ČČ,µ«æɲā³ö¼×Ķ锢ŹÆÄ«”¢ĒāĘųČ¼Éյķ“Ó¦ČČ:
CH4(g)+2O2(g)=CO2(g)+2H2O(l)£»”÷H1=-890.3kJ/mol
C(ŹÆÄ«,s)+O2(g)=CO2(g) £»¦¤H2=-393.5 kJ/mol
H2(g)+1/2O2(g)=H2O(l) £»”÷H3=-285.8 kJ/moL
ŌņÓÉŹÆÄ«ÓėĒāĘų·“Ӧɜ³É¼×ĶéµÄČČ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ__________________________________________.
 Õć“óÓÅѧŠ”ѧğ¼¶ĻĪ½Ó½Ż¾¶Õć½“óѧ³ö°ęÉēĻµĮŠ“š°ø
Õć“óÓÅѧŠ”ѧğ¼¶ĻĪ½Ó½Ż¾¶Õć½“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ĪļÖŹĄą±š | Ėį | ¼ī | ŃĪ | Ńõ»ÆĪļ | Ēā»ÆĪļ |
| »ÆѧŹ½ | ¢ŁHCl ¢Ś H2SO4»ņHNO3 H2SO4»ņHNO3 |
¢Ū NaOH»ņKOH NaOH»ņKOH ¢ÜBa£ØOH£©2 |
¢ŻNa2CO3 ¢Ž NaNO3»ņKNO3»ņK2SO4»ņNa2SO4 NaNO3»ņKNO3»ņK2SO4»ņNa2SO4 |
¢ßCO2 ¢ąNa2O |
¢įNH3 ¢āH2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾ŻŅŖĒ󊓳öĻĀĮŠ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½
(1)Ņ»¶ØĮæĒāĘųÓėĀČĘų·“Ӧɜ³ÉĀČ»ÆĒāĘųĢå,µ±Éś³É1molĒāĀČ¼üŹ±·Å³ö91.5kJµÄČČĮæ________________________________________________________.
(2)ij»Æѧ·“Ó¦µÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾,øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ(·“Ó¦ČČÓĆabc±ķŹ¾)
_____________________________________________.
(3)ij·“Ó¦µÄĘ½ŗā³£ŹżČē¹ūÓŠ1molN2 ĶźČ«·“Ó¦,ŅŖĪüŹÕČČĮæ68kJ.Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½______________________________________________.
(4)ŹµŃéÖŠ²»ÄÜÖ±½Ó²ā³öŹÆÄ«ŗĶĒāĘųÉś³É¼×Ķé·“Ó¦µÄ·“Ó¦ČČ,µ«æɲā³ö¼×Ķ锢ŹÆÄ«”¢ĒāĘųČ¼Éյķ“Ó¦ČČ:
CH4(g)+2O2(g)=CO2(g)+2H2O(l)£»”÷H1=-890.3kJ/mol
C(ŹÆÄ«,s)+O2(g)=CO2(g)£»¦¤H2=-393.5 kJ/mol
H2(g)+1/2O2(g)=H2O(l)£»”÷H3=-285.8kJ/moL
ŌņÓÉŹÆÄ«ÓėĒāĘų·“Ӧɜ³É¼×ĶéµÄČČ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ__________________________________________.
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģÕć½Ź”“ČĻŖŹŠøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©øł¾ŻŅŖĒóĢīæÕ£ŗ
£Ø1£©øł¾ŻŅŖĒ󊓳öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢ŁÓŠĖ®²Ī¼ÓµÄÖĆ»»·“Ó¦£¬³Š×÷Ńõ»Æ¼Į
¢ŚÓŠĖ®²Ī¼ÓµÄŃõ»Æ»¹Ō·“Ó¦£¬Ė®¼ČŹĒŃõ»Æ¼ĮÓÖŹĒ»¹Ō¼Į ”£
¢ŪÓŠĖ®²Ī¼ÓµÄŃõ»Æ»¹Ō·“Ó¦£®µ«Ė®¼Č²»ŹĒŃõ»Æ¼ĮÓÖ²»ŹĒ»¹Ō¼Į ”£
¢ÜÓŠĖ®²Ī¼ÓµÄø“·Ö½ā·“Ó¦ ”£
£Ø2£©”°ĀĢÉ«ŹŌ¼Į”±Ė«ŃõĖ®æÉ×÷ĪŖæóŅµ·ĻŌüĻū¶¾¼Į£¬Ļū³ż²ÉæóŅµĖŅŅŗÖŠµÄĒč»ÆĪļ£ØČēKCN£©µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗKCN+H2O2+H2O=A+NH3”ü
¢ŁÉś³ÉĪļAµÄ»ÆѧŹ½ĪŖ ”£
¢ŚŌŚ±ź×¼×“æöĻĀÓŠ0.448L°±ĘųÉś³É£®Ōņ×ŖŅʵĵē×ÓŹżĪŖ ”£
¢Ū·“Ó¦ÖŠ±»Ńõ»ÆµÄŌŖĖŲĪŖ ”£
£Ø3£©Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬Ķ¬Ģå»żµÄNH3ŗĶH2SµÄÖŹĮæ±ČŹĒ £»Ķ¬ÖŹĮæµÄNH3ŗĶH2SµÄĢå»ż±ČŹĒ £»ČōĮ½ÕßĖł¼ŖĒāŌÓŚøöŹżĻąµČ£¬NH3ŗĶH2SµÄĪļÖŹµÄĮæ±ČŹĒ ”£
£Ø4£©½«±ź×¼×“æöĻĀµÄNH3£Øg£©VLČÜÓŚĖ®ÖŠ£¬µĆµ½ĆܶČĪŖb g”¤cm-3µÄ°±Ė®a g£¬“ĖŹ±ĪļÖŹµÄĮæÅضČĪŖc mol”¤L-1£¬ŌņČÜÓŚĖ®ÖŠµÄNH3£Øg£©µÄĢå»żVŹĒ L£ØÓĆa”¢b”¢cµČ±ķŹ¾£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğ±±¾©ŹŠĆÜŌʶžÖŠø߶žĻĀĘŚ3ŌĀ·ŻŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
øł¾ŻŅŖĒ󊓳öĻĀĮŠ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½
(1)Ņ»¶ØĮæĒāĘųÓėĀČĘų·“Ӧɜ³ÉĀČ»ÆĒāĘųĢå,µ±Éś³É1molĒāĀČ¼üŹ±·Å³ö91.5kJµÄČČĮæ________________________________________________________.
(2)ij»Æѧ·“Ó¦µÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾,øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ(·“Ó¦ČČÓĆabc±ķŹ¾)
_____________________________________________.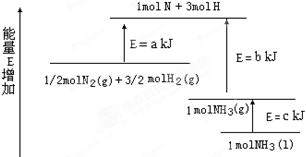
(3)ij·“Ó¦µÄĘ½ŗā³£Źż Čē¹ūÓŠ1molN2ĶźČ«·“Ó¦,ŅŖĪüŹÕČČĮæ68kJ.Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½______________________________________________.
Čē¹ūÓŠ1molN2ĶźČ«·“Ó¦,ŅŖĪüŹÕČČĮæ68kJ.Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½______________________________________________.
(4)ŹµŃéÖŠ²»ÄÜÖ±½Ó²ā³öŹÆÄ«ŗĶĒāĘųÉś³É¼×Ķé·“Ó¦µÄ·“Ó¦ČČ,µ«æɲā³ö¼×Ķ锢ŹÆÄ«”¢ĒāĘųČ¼Éյķ“Ó¦ČČ:
CH4(g)+2O2(g)=CO2(g)+2H2O(l)£»”÷H1=-890.3kJ/mol
C(ŹÆÄ«,s)+O2(g)=CO2(g) £»¦¤H2="-393.5" kJ/mol
H2(g)+1/2O2(g)=H2O(l) £»”÷H3="-285.8" kJ/moL
ŌņÓÉŹÆÄ«ÓėĒāĘų·“Ӧɜ³É¼×ĶéµÄČČ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ__________________________________________.
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com