
| ���� |
| ����ɸ |
| ����ɸ |
| 1��0.9��0.8 |
| 22.4 |
| ���� |
| ����ɸ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
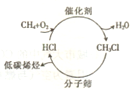
 ����ʯ����Դ���������ţ���Ȼ����Դ��ת������Խ��Խ�ܵ����ǵĹ�ע����ͼ��ʾ������з�������Ȼ��������;�������ȣ������ڴ��������·��������Ȼ���Ӧ������һ�ȼ��飻Ȼ��һ�ȼ�����400���������ͨ������ɸ��ת��Ϊ��̼ϩ����
����ʯ����Դ���������ţ���Ȼ����Դ��ת������Խ��Խ�ܵ����ǵĹ�ע����ͼ��ʾ������з�������Ȼ��������;�������ȣ������ڴ��������·��������Ȼ���Ӧ������һ�ȼ��飻Ȼ��һ�ȼ�����400���������ͨ������ɸ��ת��Ϊ��̼ϩ����| ����ɸ |
| ����ɸ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��̩�ݶ��и߶���һ�Σ�10�£���ʱ��ҵ��ѧ�Ծ��������棩 ���ͣ������
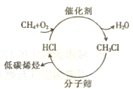
(14��)����ʯ����Դ���������ţ���Ȼ����Դ�Ŀ��������ܵ�Խ��Խ��Ĺ�ע������Ȼ������Ҫ�ɷ�CH4��Ϊԭ�Ͼ��ϳ�������Ҫ�ɷ�CO��H2���ƻ�ѧƷ����Ŀǰ��Ȼ��ת�����õ���Ҫ����·�ߡ����������͡�ú����̿Ϊԭ���ƺϳ����������ʻ�����Fe(CO)5�ݵȶ������Ժϳ���Ϊԭ�Ϻϳɼ״��ͺϳɰ������������еĴ��������ж���
���ʴ��������⣺
��1��Fe(CO)5�����Ļ��ϼ�Ϊ0��д����ԭ�ӵĻ�̬�����Ų�ʽ������������������
��2��ԭ����Ŀ�͵�����������۵�����������ͬ������Ϊ�ȵ����壬�ȵ�����������ƵĽṹ��������CO���ӻ�Ϊ�ȵ�����ķ��Ӻ����ӷֱ�Ϊ�������������������ѧʽ����CO���ӵĽṹʽ�ɱ�ʾ����������������
��3��Fe(CO)5�����³�Һ̬���۵�Ϊ��20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)5����Ϊ�����������������塣
��4����CH4��CO��CH3OH�У�̼ԭ�Ӳ�ȡsp3�ӻ��ķ�������������������������CH3OH���ۡ��е��CH4���ۡ��е�ȸߣ�����Ҫԭ��������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�Ͼ��и�����ѧ�����п��Ի�ѧ���� ���ͣ�������
��12�֣�����ʯ����Դ���������ţ���Ȼ����Դ��ת������Խ��Խ�ܵ����ǵĹ�ע����ͼ������з�������Ȼ��������;�������ȼ����ڴ��������·��������Ȼ���Ӧ������һ�ȼ��飻Ȼ��һ�ȼ�����400��C��������ͨ������ɸ��ת��Ϊ��̼ϩ����
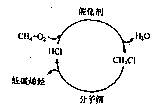
�����������Ϣ�ش��������⣺
��1����һ�ִη�ӦͶ��ʱ������Ȼ�����������ȣ���ͬ������Ϊ ��
��2����һ�ȼ������ɱ�ϩ�Ļ�ѧ����ʽΪ ��
��3������ѷ�ӦͶ�ϱȣ�����;���еĵ�һ�����������Ȼ���Ӧ����ת����Ϊ80%������ˮ�������������е�һ�ȼ����ʣ��CH4��O2��HClȫ�����ò�Ͷ��ڶ��ִ��������ڶ���һ�ȼ��鷴Ӧ���ɺ�����ϩ����ϩ����ϩ���Ȼ���Ļ�����壬̼ԭ�ӵ�������Ϊ90%����������е�ϩ�������ʣ��HClҲȫ�����ò�Ͷ��ڶ��ִ���������
�ٱ�״���£�1m3���龭��һ�ַ�Ӧ�ɵõ� kg��ϩ����ϩ����ϩ�Ļ�
�����塣
��Ϊ�����һ�ִεõ��ĵ����ĵ�̼ϩ�����ڶ��ִη�ӦͶ��ʱ���貹���CH4��
O2��HCl�������Ϊ����ͬ������ ������д���ٺ͢ڵļ�����̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʯ����Դ���������ţ���Ȼ����Դ�Ŀ��������ܵ�Խ��Խ��Ĺ�ע������Ȼ������Ҫ�ɷ���CH4��Ϊԭ�Ͼ��ϳ�������Ҫ�ɷ�ΪCO��H2���ƻ�ѧƷ����Ŀǰ��Ȼ��ת�����õ���Ҫ����·�ߡ����������͡�ú����̿Ϊԭ���ƺϳ����������ʻ�����Fe(CO)5�ݵȶ������Ժϳ���Ϊԭ�Ϻϳɼ״��ͺϳɰ������������еĴ��������ж�����ش��������⣺
��1����Fe (CO)5�������Ļ��ϼ�Ϊ0��д����ԭ�ӵĻ�̬�����Ų�ʽ��_________________��
��2����CO��Ϊ�ȵ�����ķ��Ӻ����ӷֱ�Ϊ___________��___________������һ�ּ��ɣ��ѧʽ����CO���ӵĵ���ʽΪ__________��CO���ӵĽṹʽ�ɱ�ʾ��______ _��
��3����CH4��CO��CH3OH�У�̼ԭ�Ӳ�ȡsp3�ӻ��ķ�����_________________��CH3OH���ۡ��е��CH4�ߣ�����Ҫԭ����_____________________________________��
��4��CH3CHO�����У���CH3�е�̼ԭ�Ӳ���____________�ӻ���ʽ����CHO�е�̼ԭ�Ӳ�ȡ_____________�ӻ���ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com