



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | C6H5ONa |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 | 11.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʯ���ѻ��õ���̬ϩ�� | B��ʯ���ѽ�Ŀ������ת�������� |
| C���ҹ����������䡱���̵���ָ���� | D��ˮú����ͨ��ú��Һ���õ�������ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��ij������Һ��ֻ��NH ��Cl����H����OH���������ӣ���Һ��һ�����ڣ� ��Cl����H����OH���������ӣ���Һ��һ�����ڣ�c(Cl��)>c(NH  )>c(H��)>c(OH��) )>c(H��)>c(OH��) |
| B�����ʵ���Ũ����ͬ��4����Һ����CH3COONa����NaNO3����Na2CO3����NaOH�� pH�Ĵ�С˳���ǣ���>��>��>�� |
C����Na2CO3��NaHCO3�Ļ����Һ�У�c(Na��)��c(H��)��c(HCO )��c(OH��)��c(CO )��c(OH��)��c(CO ) ) |
| D��25��ʱ��pH��10��CH3COONa��Һ��pH��10�İ�ˮ�У���ˮ�������c(OH��)֮��Ϊ1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȼ���Ƿ��Ӿ��壬�۷е�� |
| B��CH4���ӽṹ�ǶԳƵ� |
| C��H2O�ǷǼ��Է��� |
| D��ʹ�ÿ�ȼ�����ú��ʯ�ͣ����Խ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
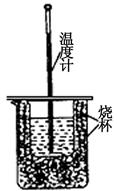
| ʵ����� | ��ʼ�ζ��ܶ��� | �յ�ζ��ܶ��� |
| 1 | 0.00mL | 24.04mL |
| 2 | 0.50mL | 24.46mL |
| 3 | 2.50mL | 25.02mL |
�鿴�𰸺ͽ���>>
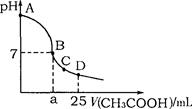
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��V��NaOH��="=" 0ʱ��c��H+��W��="=" 1 �� 10��2 mol��L |
B��V��NaOH��< 10 mLʱ�������ܴ���c��Na����="=" 2 c��C2O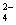 ��+ c��HC2O ��+ c��HC2O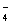 �� �� |
| C��V��NaOH��="=" 10 mLʱ��c��H+��W��="=" 1 �� 10��7mol��L |
D��V��NaOH��> 10 mLʱ��c��Na����> c��C2O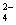 ��>c��HC2O ��>c��HC2O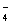 �� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
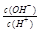 =1��10-8������������ȷ����
=1��10-8������������ȷ����| A����Һ��ˮ�������c(H+)��10-10mol/L |
| B������Һ�д���ĵ����Ϊ1% |
| C����Һ�м���һ����CH3COONa������ˮϡ�ͣ���Һ��c(OH-)������ |
| D����Һ��c(H+)��c(CH3COO-)��0.1 mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����1 mL 1.0��10-5 mol/L����ϡ�͵�1000 mL���õ�pH��8 ������ |
| B����1 mL pH��3��һԪ����Һϡ�͵�10 mL������Һ��pH��4�������Ϊ���� |
| C����pH=1������ֱ��к�1 mL pH="13" NaOH��Һ�Ͱ�ˮ��NaOH�������������� |
| D��pH��2��������pH��1������Ƚϣ�2 c(Cl�� )��c(SO42�� ) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com