
��ͼ�ǹ�ҵ��������淋����̡�
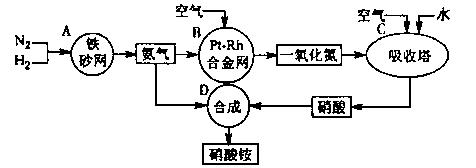
��1��������C��ͨ�������Ŀ���� ��
A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ���� ������ĸ����
��2����֪��4NH3��g��+3O2��g��=2N2��g��+6H2O��g�� ��H=һ1266��8kJ��mol
N2��g��+O2��g��=2NO��g�� ��H=+180��5 kJ��mol
д�������´��������Ȼ�ѧ����ʽ�� ������������Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ��
��3����֪��N2��g��+3H2��g�� ![]() 2NH3��g�� ��H=һ92 kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��������ĸ��
2NH3��g�� ��H=һ92 kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��������ĸ��
A�������¶� B��ʹ�ô��� C������ѹǿ
D��ѭ�����úͲ��ϲ��䵪�� E����ʱ�Ƴ���
��4����һ���¶Ⱥ�ѹǿ�£���H2��N2��3��1������ȣ����ܱ������л�ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������NH3�������������Ϊ17��6������ʱH2��ת����Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ͼ�ǹ�ҵ��������淋����̡�
��1��������C��ͨ�������Ŀ���� ��
A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ���� ������ĸ����
��2����֪��4NH3��g��+3O2��g��=2N2��g��+6H2O��g�� ��H = ��1266��8kJ��mol
N2��g��+O2��g��=2NO��g�� ��H= +180��5 kJ��mol
д�������´��������Ȼ�ѧ����ʽ�� ������������Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ��
��3����֪�� ��H= �� 92 kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��������ĸ��
A�������¶� B��ʹ�ô��� C������ѹǿ
D��ѭ�����úͲ��ϲ��䵪�� E����ʱ�Ƴ���
��4����һ���¶Ⱥ�ѹǿ�£���H2��N2��3��1������ȣ����ܱ������л�ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������NH3�������������Ϊ17��6������ʱH2��ת����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012ѧ��ɽ��ʡΫ���и߶�������ҵ��ѧ�Ծ����ģ� ���ͣ������
��ͼ�ǹ�ҵ��������淋����̡�
 (1)������C��ͨ�˿�����Ŀ����
(1)������C��ͨ�˿�����Ŀ����
A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ���� (����ĸ)��
(2)��֪��4NH3(g)+3O2(g)=2N2(g)+6H2O(g)����H=-1266��8kJ��mol
N2(g)+O2(g)=2NO(g)����H=+180.5kJ��mol
д�������´��������Ȼ�ѧ����ʽ��
(3)��֪��N2(g)+3H2(g)  2NH3(g)����H=-92kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��(����ĸ)
2NH3(g)����H=-92kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��(����ĸ)
A�������¶� ���� B��ʹ�ô��� ���� C������ѹǿ
D��ѭ�����úͲ��ϲ��䵪�� �������� E����ʱ�Ƴ���
(4)��һ���¶Ⱥ�ѹǿ�£���H2��N2��3��1(�����)���ܱ������л�ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������NH3�������������Ϊ25������ʱH2��ת����Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��㶫ʡ����12���¿����ۻ�ѧ�Ծ��������棩 ���ͣ������
��I����ͼ�ǹ�ҵ��������淋����̡�

��1��������C��ͨ�������Ŀ���� ��A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ���� ������ĸ����
��2����֪��4NH3(g) + 3O2(g) = 2N2(g) +6H2O(g) ��H =��1266.8kJ/mol
N2(g) + O2(g) = 2NO(g) ��H = +180.5 kJ/mol
д�������´��������Ȼ�ѧ����ʽ��
��II��ij����С��ͬѧ��ͭƬ����ϡ���ᣬ���ֿ�ʼʱ��Ӧ�dz�����һ��ʱ���Ӧ�������Լӿ졣��С��ͨ��ʵ��̽����ԭ��
��3���÷�Ӧ�����ӷ���ʽΪ___________________________________________________��
��4������������衣��ʵ���з�Ӧ�������Լӿ��ԭ�������_____________________��
A����Ӧ���ȵ����¶����� B��ѹǿ����
C���������д����� D����Ӧ��Ӵ��������
��5������̽�����ⶨ��Ӧ��������Һ��ͬʱ����¶ȣ�������±���
|
ʱ��/min |
0 |
5 |
10 |
15 |
20 |
25 |
35 |
50 |
60 |
70 |
80 |
|
�¶�/�� |
25 |
26 |
26 |
26 |
26 |
26 |
26.5 |
27 |
27 |
27 |
27 |
���ʵ��Ŀ�ĺͱ������ݣ���ó��Ľ�����__________________________________��
��6����һ��̽�������������˽��ѧ��Ӧ�IJ�����м������ܶԷ�Ӧ�д����ã����������ʵ����Ʊ�����ʵ��Ŀ�IJ���������
|
ʵ�� ��� |
ͭƬ ����/g |
0.1mol��L-1�� �������/mL |
����ͭ ����/g |
�������� ����/g |
ʵ��Ŀ�� |
|
�� |
5 |
20 |
_______ |
_______ |
ʵ��ٺ͢�̽��_________��Ӱ�죻ʵ��ٺ͢�̽�����������Ӱ�졣 |
|
�� |
5 |
20 |
0.5 |
0 |
|
|
�� |
5 |
20 |
0 |
0.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�����и�����ѧ��ѧ����飨������ѧ�Ծ� ���ͣ������
��ͼ�ǹ�ҵ��������淋����̡�

��1��������C��ͨ�������Ŀ���� ��
A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ���� ������ĸ����
��2����֪��4NH3��g��+3O2��g��=2N2��g��+6H2O��g�� ��H= -1266��8kJ��mol
N2��g��+O2��g��=2NO��g�� ��H=+180��5 kJ��mol
д�������´��������Ȼ�ѧ����ʽ�� ��
����������Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ��
��3����֪��N2��g��+3H2��g��
 2NH3��g�� ��H=һ92 kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ��
��������ĸ��
2NH3��g�� ��H=һ92 kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ��
��������ĸ��
A�������¶� B��ʹ�ô��� C������ѹǿ
D��ѭ�����úͲ��ϲ��䵪�� E����ʱ�Ƴ���
��4����һ���¶Ⱥ�ѹǿ�£���H2��N2��3��1������ȣ����ܱ������л�ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������NH3�������������Ϊ33��33������ʱH2��ת����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12�֣���ͼ�ǹ�ҵ��������淋����̡�

��1��������C��ͨ�������Ŀ���� ��A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ���� ������ĸ����
��2����֪��4NH3��g��+3O2��g��=2N2��g��+6H2O��g�� ��H=һ1266��8kJ��mol
N2��g��+O2��g��=2NO��g�� ��H=+180��5 kJ��mol
д�������´��������Ȼ�ѧ����ʽ�� ������������Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ��
��3����֪��N2��g��+3H2��g�� 2NH3��g�� ��H= һ92 kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��������ĸ��
2NH3��g�� ��H= һ92 kJ��mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ�� ��������ĸ��
A�������¶� B��ʹ�ô��� C������ѹǿ
D��ѭ�����úͲ��ϲ��䵪�� E����ʱ�Ƴ���
��4����һ���¶Ⱥ�ѹǿ�£���H2��N2��3��1������ȣ����ܱ������л�ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������NH3�������������Ϊ17��6������ʱH2��ת����
Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com