
����Ļ�ѧʽ����CaxMgySipO22(OH)2��ʾ��Ҳ����Ca��Mg��Si��H���������ʾ����
��1��ȡ8.l0g�����ĩ���������أ����������0.18g��������Ħ������Ϊ______��
��2����ȡ4.05g�����ĩ����l mol/L������l00mL�г���ܽ⣬���յò���������2. 40g.���ˣ�����Һ��ϴ��Һ�ϲ��������м�����������м���õ�����336mL(��״����).��
��p=_________��
�ڰ���Ļ�ѧʽ�������������ʽ����ʾΪ________��
��1��810g��mol-1����2��8��2CaO��5MgO��8SiO2��H2O
���������������1���ɰ���Ļ�ѧʽCaxMgySipO22(OH)2���Կ���ÿ������������ʱ������Ӧ��ֻ������1��ˮ���ӡ�n(H2O)=m/M=0.18g��18g/mol=0.01mol.,����������ʵ���Ҳ��1mol���������Ħ������Ϊ8.10g��0.01mol=810g/mol.��2��n(����)= 4.05g��810g/mol=0.005mol.���к���Si�����ʵ���Ϊ��2. 40g. ��60g/mol=0.04mol..���ÿĦ���İ����к���Si�����ʵ���Ϊ0.04mol��0.005mol=8.�ɷ���ʽFe+2HCl=FeCl2+H2����֪��n(H2)=0.336L��22.4L/mol=0.015mol.��������Ӧ���������������ʵ���Ϊ0.03mol.�������ĵ�HCl�����ʵ���Ϊ1mol/L��0.1L-0.03mol=0.07mol.��ôÿĦ���İ���Ӧ��Ҫ���ĵ�HCl�����ʵ���Ϊ0.07mol��0.005mol=14������Ca��Mg����+2�۵Ľ��������������ᷴӦʱÿ�����������Ӷ�Ҫ���2��Cl-.����n(Ca2+)+n(Mg2+)=14��2=7.��X+Y=7.�ٽ����Է�������40X+24Y+28��8+16��22+17��2=810.��ʽ������⡣�ɵ�X=2��Y=5�����Ըû�����ķ���ʽΪCa2Mg5Si8O22(OH)2. �����������ʽ����ʾΪ2CaO��5MgO��8SiO2��H2O.
���㣺��������Ħ�������ļ��㡢�����εĻ�ѧʽ��ȷ���������������ʽ�ı�ʾ��֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�ǵ�Ԫ�صļ��ּ�̬���������Ķ�Ӧ��ϵ��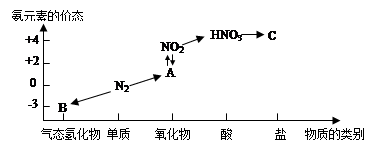
�ش��������⣺
��1��д��N2��һ����; ��
��2����NԪ�ػ��ϼ۷�����N2���������Ժͻ�ԭ�ԡ�����һ��˵�����û�ѧ����ʽ��ʾ��
�������� ��
��ԭ�� ��
��3��HNO3����ͼ�е�����C�����ڼ���Cl-�Ĵ��ڣ���C�Ļ�ѧʽΪ________��
��4��ʵ������ȡ����B�Ļ�ѧ����ʽΪ ��
��5��NO2��ˮ��Ӧ��������A�Ļ�ѧ����ʽΪ ��
��6��Ũ������ľ̿�ڼ��������·�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ӻ�����AB2�����������ӵĵ��Ӳ�ṹ��ͬ��1 mol AB2�к�54 mol���ӣ��������з�Ӧ��
��H2��B2 C
C
��B2��X��Y��AB2��H2O
��Y��C��AB2��Z��Z��Ư�����á�
�������������ش��������⣺
��1��д���������ʵĻ�ѧʽ��AB2__________��X________��Y________��Z________��
��2���õ���ʽ��ʾAB2���γɹ��̣�_____________________________________________��
��3��д����Ӧ�ڵĻ�ѧ����ʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ú��һ�ֳ��õ�ȼ�ϡ�����úȼ�ղ����ķ����к���SO2��NO2��NO�ȶ����к�������̳�����Ի��������Ⱦ�������Ҫ��ú���мӹ�����ȼ�գ��������������ŷš�
��1����úֱ�ӽ���ȼ�գ���ɻ�����Ⱦ����Ҫԭ��֮һ���γ����������ꡣ
��д���й�����ת��Ϊ����Ļ�ѧ����ʽ____________________________��
�������Ƕ���������������ۣ�������ȷ����_________________________���������գ���
a.ɱ��ˮ�еĸ��������������ʳ����Դ���ƻ�ˮ����̬ϵͳ
b.�Ե��ߡ����졢���������ݵȾ������������
c.�ƻ�������
d.�����������еĿ����ʷ�������ת��Ϊ�����Σ���ֲ���ṩ����
��2����ͼ�Ƕ�úȼ�ղ����ķ������г����������Ļ�������ʾ��ͼ����д���ڷ��������γɸ�����Ĺ���������������Ҫ��ѧ��Ӧ�Ļ�ѧ����ʽ_______��
��3�������Ժ��ڷ����к����������ӵIJ��������ǣ�д�������ƣ�_______�����Ի����ĸ���Ӱ����____________________________________________��
��4�����������������Ƿ���SO2�ļ�����_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���п�ͼ�漰����������Ԫ����,��һ��Ԫ����,�����Ϊ1��18��Ԫ�ء�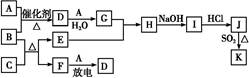
��֪:A��FΪ��ɫ���嵥��,BΪ���д̼�����ζ������,CΪ��ɫ������,EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ�г�������ش���������:
��1��D�Ļ�ѧʽΪ����������������;F�ĽṹʽΪ�� ������
��2��A��B��Ӧ�Ļ�ѧ����ʽΪ�� ��
��3��E��G��ϡ��Һ��Ӧ�����ӷ���ʽΪ�� ��
��4��B��C��Ӧ�Ļ�ѧ����ʽΪ�� ��
��5��J��K��ͬ�ֽ����IJ�ͬ�Ȼ���,KΪ��ɫ������д��SO2��ԭJ����K�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CO2��Ŀǰ�����к�����ߵ�һ���������塣��ˣ����ƺ�����CO2�ǽ������ЧӦ����Ч;����
��1�����й���CO2��˵����ȷ���ǣ�����ţ�_________��
�ټ��ٻ�ʯȼ�ϵ�ʹ�ã��������̫���ܡ����ܵ������Դ����Ч���ʹ�����CO2�ĺ���
��ֲ�����֣�����ֲ�����������Ч���ʹ�����CO2�ĺ���
�۶������������������̼���������ĺ������ǿ��������ձ�������
�ܿ�����CO2�ĺ������ᵼ��������γ�
��2�����д�ʩ���ܼ��ٶ�����̼�ŷŵ��ǣ�����ţ� _________��
������̫��������
�ڹ�ͣС�����ҵ
�۾��С�����һСʱ��Ϩ�ƻ
���ƹ�ʹ��úҺ������
��3�����з�Ӧ����������������ǣ�����ţ�_________��
���ô����Ʋ��� ����ú̿��ȼ��
��������ʯ���� ���ð���̼���
��4��Ŀǰ�����ڶ�����̼�Ƿ�Ϊ������Ⱦ���в�ͬ�Ĺ۵㡣��Ϊ��������̼���Ǵ�����Ⱦ��������ǣ�����ţ�_________��
�ٶ�����̼����Ҫ�Ļ���ԭ��
�ڶ�����̼��ֲ�������õıر�ԭ��
�۶�����̼����ɫ����ζ����������
�ܳ�������̼���⣬���顢һ����������Ҳ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�輰�仯����������ִ������ķ�չ��������ס���ش������й����⣺
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
�ٳ�����Ͽˮ���ӡ���ʯӢ���ά�����մ�����
����ͨ�������ݹ�̫���ܵ��
| A���٢ڢ� | B���ۢܢ� | C���ڢۢ� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ʯʯ�ޡ���Ҫ�ɷ�����������������þ���ᾧˮ�����Ļ�ѧʽ��Mg6[(OH)4Si2O5]2��
��1��������ʯʯ�ޡ�����������ʽΪ______________________������ԭ�Ӱ뾶����Ԫ��
�����ڱ��е�λ����______________________��
��2��Siԭ�ӵ������ĵ����Ų�ʽΪ_____________��SiO2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
Ϊ________________________________________________��
��3�� SiCl4��SiO2���۵�_____(��͡������ߡ�)��ԭ����__________________________��
��4������Щ���治���ж�Si��O�ķǽ�����ǿ�� ��
| A������Si��O�����ڱ��е�λ�� |
| B��SiO2��ˮ��������Ӧ |
| C��Si��һ����������������Ӧ������SiO2 |
| D��H2SiO3�����Ա�H2O������ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ڿƼ���������Ӧ�ù㷺��
��1����ҵ�ϳ�����ʯ�Һ�������Ӧ��ȡƯ�ۣ���ѧ����ʽ�� ��
��2��ʵ������MnO2��Ũ���ᷴӦ��ȡ������ԭ�����£�MnO2 + 4HCl MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O
������ȡ11.2 L Cl2����״������������Ӧ����MnO2������Ϊ______g��
����ƽ���ƶ�ԭ�����Ϳ����ű���ʳ��ˮ���ռ�������ԭ�� ������ϱ�Ҫ�Ļ�ѧ���P���ֻش�
���Ʊ�����ʱ������NaOH��Һ����β���������Լ�Ҳ������������������____������ĸ����
a. KI��Һ b. FeCl2��Һ c. KCl��Һ
д����ѡ��������Լ���Cl2��Ӧ�����ӷ���ʽ��_______��
��Ҳ����Ũ��ˮ����������ͬʱ����һ������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽ��_______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com