���
�⣺����ϩ�ĽṹʽΪ
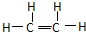
����ϩ��̼���ϸߣ�ȼ�շ���������ӦC
2H
4+3O
22CO
2+2H
2O����������̣���ϩ���Ȼ�����һ�������·����ӳɷ�Ӧ���������飬����ʽΪCH
2=CH
2+HCl��CH
3CH
2Cl������Һ���ڴ��������·�Ӧ
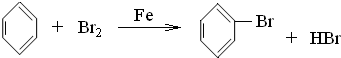
�����屽���÷�ӦΪȡ����Ӧ���������ƿ��Ժ��嵥�ʷ�ӦBr
2+2NaOH�TNaBr+NaBrO+H
2O�����廯�ơ��������Ƶ�ˮ��Һ�����屽�����ܣ������ٷ�Һ�����룻
�ʴ�Ϊ��
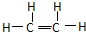
���к��̣�������Ӧ��CH
2=CH
2+HCl��CH
3CH
2Cl��
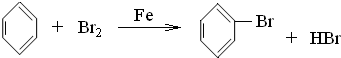
���������ƣ�Br
2+2NaOH�TNaBr+NaBrO+H
2O��
����ͬ��ͬѹ�£�������������������ܶ�Ϊ13���ʻ�������ƽ����Է�������Ϊ13��2=26����ϩ������Է���������СΪ28��C
2H
4����С��26��һ��Ϊ������������һ��ΪCH
4�������4.48L�����������ʵ���Ϊ��n=
=
=0.2mol���ʻ�������������Ϊ0.2mol��26g/mol=5.2g����ˮ����2.8g��ӦΪϩ��������������n��CH
4��=
=0.15mol��ϩ�������ʵ���Ϊ0.2mol-0.15mol=0.05mol����ϩ������Է�������ΪM=
=
=56g/mol��
�ʴ�Ϊ��56g/mol��
��1������ϩ����������ʵ����ֱ�Ϊxmol��ymol�����ݶ������ʵ���֮����Hԭ���غ��з��̣���x+y=1��4x+6y=
����ã�x=0.5��y=0.5��
��n��C
2H
4����n��C
2H
6��=0.5mol��0.5mol=1��1��
�ʴ�Ϊ��1��1��
��2������̼Ԫ���غ��֪����Ӧ��CO��CO
2�����������ʵ���֮��Ϊ1mol��2=2mol����
b=2�����b=3��
�Ӧ��CO��CO
2�����ʵ����ֱ�Ϊamol��bmol�����ݶ���֮������ԭ���غ��з��̣���a+b=2��a+2b=3��2-
����ã�a=0.5��b=1.5����n��CO����n��CO
2��=0.5mol��1.5mol=1��3��
�ʴ�Ϊ��3��1��3��
��3������ˮ�����ʵ���=
=2.5molֻ����ϩʱ��aֵ�����Hԭ���غ��֪��a�ļ���ֵΪ
=
mol��ֻ������ʱ��aֵ��С������Hԭ���غ��֪��a�ļ�СֵΪ
=
mol����a��ȡֵ��Χ��
��a��
��
�ʴ�Ϊ��
��a��
��

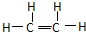 ����ϩ��̼���ϸߣ�ȼ�շ���������ӦC2H4+3O2
����ϩ��̼���ϸߣ�ȼ�շ���������ӦC2H4+3O2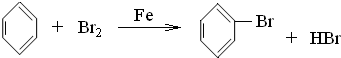 �����屽���÷�ӦΪȡ����Ӧ���������ƿ��Ժ��嵥�ʷ�ӦBr2+2NaOH�TNaBr+NaBrO+H2O�����廯�ơ��������Ƶ�ˮ��Һ�����屽�����ܣ������ٷ�Һ�����룻
�����屽���÷�ӦΪȡ����Ӧ���������ƿ��Ժ��嵥�ʷ�ӦBr2+2NaOH�TNaBr+NaBrO+H2O�����廯�ơ��������Ƶ�ˮ��Һ�����屽�����ܣ������ٷ�Һ�����룻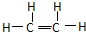 ���к��̣�������Ӧ��CH2=CH2+HCl��CH3CH2Cl��
���к��̣�������Ӧ��CH2=CH2+HCl��CH3CH2Cl��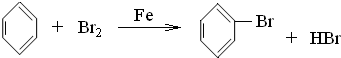 ���������ƣ�Br2+2NaOH�TNaBr+NaBrO+H2O��
���������ƣ�Br2+2NaOH�TNaBr+NaBrO+H2O��

 ��У����ϵ�д�
��У����ϵ�д�
 ������ʾA��B��C��D��E�仯��ϵ������ʡȥ�˷�Ӧ����������
������ʾA��B��C��D��E�仯��ϵ������ʡȥ�˷�Ӧ���������� 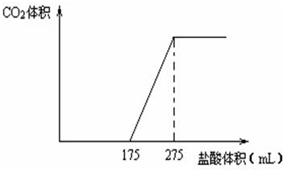 ȡNaHCO3��Na2O2�Ĺ�������x g����һ�ܱ������м�����250�棬��ַ�Ӧ���ų��������壮����Ӧ��Ĺ���ֳ���ȫ��ͬ�����ݣ�������һ��Ͷ�뵽������BaCl2��Һ�У����ɵõ�3.94g��������һ������������ˮ��������ų�������ˮ��Һ�л�����μ���ij���ʵ���Ũ�ȵ����ᣬ���������������������֮��Ĺ�ϵ����ͼ��ʾ���Իش��������⣺
ȡNaHCO3��Na2O2�Ĺ�������x g����һ�ܱ������м�����250�棬��ַ�Ӧ���ų��������壮����Ӧ��Ĺ���ֳ���ȫ��ͬ�����ݣ�������һ��Ͷ�뵽������BaCl2��Һ�У����ɵõ�3.94g��������һ������������ˮ��������ų�������ˮ��Һ�л�����μ���ij���ʵ���Ũ�ȵ����ᣬ���������������������֮��Ĺ�ϵ����ͼ��ʾ���Իش��������⣺ ��1����֪����298K��1.01KPa�£����ȶ��ĵ��ʷ�����Ӧ����1mol��̬������ķ�Ӧ�Ƚиû�����������ȣ���H������ͼ��ʾΪ����Ԫ���⻯��a��b��c��d������������ʾ��ͼ���Իش��������⣺������ɳ��ǽ���Ԫ���⻯����ȶ������䷴Ӧ�ȡ�H�Ĺ�ϵ
��1����֪����298K��1.01KPa�£����ȶ��ĵ��ʷ�����Ӧ����1mol��̬������ķ�Ӧ�Ƚиû�����������ȣ���H������ͼ��ʾΪ����Ԫ���⻯��a��b��c��d������������ʾ��ͼ���Իش��������⣺������ɳ��ǽ���Ԫ���⻯����ȶ������䷴Ӧ�ȡ�H�Ĺ�ϵ