
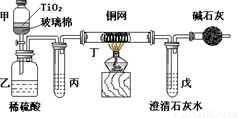
. ��Ƴ������������ƹ��գ����������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN������������CNO�����������������¼�����NaClO������N2��CO2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ���ͨ���ⶨ������̼����ȷ��CN���������İٷ��ʡ�

��Ũ����CN�����ӵ���ˮ�����NaClO��Һ�Ļ��Һ��200 mL������CN����Ũ��Ϊ0.05 mol��L��1��
������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺
�� ���з�Ӧ�����ӷ���ʽΪ ��
���з�Ӧ�����ӷ���ʽΪ ��
�� �������ɵ������N2��CO2�⣬����HCl��������Cl2�ȡ����м���ij����Լ��DZ���ʳ��ˮ���������� ��
����ʵ���е������� ��
װ�м�ʯ�ҵĸ���ܵ������� ��
�� ����ʢ�к�Ca(OH)2 0.02mol��ʯ��ˮ����ʵ�������й�����0.82 g���������ʵ���в��CN���������İٷ��ʵ��� ���ò��ֵ�빤ҵʵ�ʴ����İٷ����������ƫ�ͣ���Ҫ˵�����ܵ�ԭ�� ��
�� ��
�����һ�������ȷ�ȵĽ��飨Ҫ�пɲ����ԣ��Ҳ�����
��
(��15��)
�� CN��+ClO��CNO��+Cl����
2CNO��+2H+ +3ClO��N2��+2CO2��+3Cl��+H2O
�� ��ȥHCl���� ȥ��Cl2 ��ֹ������CO2��������Ӱ��ⶨȷ��
�� 82%
��װ���ҡ��������п���������CO2��
��CO2�������ٶȽϿ�δ�����еij���ʯ��ˮ��ַ�Ӧ ��
��Cl2��HCl�ڱ�������δ������ȫ��
(��������������㼴�ɣ�ÿ���һ���1�֣���2��)
����һ������������Һһ��ȫ���������У���Ϊ�ִμ��룬����CO2�IJ����ٶȣ�
�����������ƿ����Ϊ�����������ӵ��Ǹ����в���һ�����ܵ�Һ�����£���Ӧ������ͨ���ȥCO2�Ŀ�����ʹװ����������CO2�����ܶ����Ca(OH)2��Ӧ��
�������������г���ʯ��ˮ��ΪŨ�Ƚϴ��NaOH��Һ����Ӧ�����������м�������CaCl2�������������ȵ�
(�����������һ�㼴��) ��ÿ��2�֣���15�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��Ũ����CN -����ˮ�����NaClO��Һ�Ļ��Һ��200 mL(����CN -��Ũ��Ϊ0.05 mol�� L-1)������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺?
(1)���з�Ӧ�����ӷ���ʽΪ ��?
���з�Ӧ�����ӷ���ʽΪ ��
(2)�������ɵ������N2��CO2�⣬����HCl��������Cl2�ȣ�����ʵ����ͨ���ⶨ������̼������ȷ����CN-�Ĵ���Ч��������м���ij����Լ��ǡ� (����ĸ)?
a.����ʳ��ˮ b.����NaHCO3��Һ? c.ŨNaOH��Һ d.Ũ����?
(3)����ʵ���е������� ��?
װ�м�ʯ�ҵĸ���ܵ������� ��?
(4)����ʢ�к�Ca(OH)2 0.02 mol��ʯ��ˮ����ʵ�������й�����0.82 g���������ʵ���в��CN-�������İٷ��ʵ��� ��?
��˵���ò��ֵ��ʵ�ʴ����İٷ������ƫ����ƫ�� ����Ҫ˵�����ܵ�ԭ�� ��?
(5)�����һ�������ȷ�ȵĽ���(Ҫ�пɲ����ԣ�����ʹ������ù��ڸ���) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�����е���У������ѧ������������ѧ�Ծ��������棩 ���ͣ�ʵ����
��Ƴ������������ƹ��գ������ŷŵķ�ˮ�к��еľ綾CN-���ӣ����������ƹ�����������������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN-����������OCN-���������������¼�����NaClO������N2��CO2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ����ⶨCN-�������İٷ��ʡ���Ũ����CN-���ӵ���ˮ�����NaClO��Һ�Ļ��Һ��200mL������CN-��Ũ��Ϊ0.05mol��L-1��������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺
��1�����з�Ӧ�����ӷ���ʽΪ ��
��2���������ɵ������N2��CO2�⣬���и�����HCl��Cl2�ȣ�����ʵ����ͨ���ⶨ������̼������ȷ����CN-�Ĵ���Ч��������м���ij����Լ���___________������ĸ��
A������ʳ��ˮ B������NaHCO3��Һ C��ŨNaOH��Һ D��Ũ����
��3������ʵ���е������� ��
װ�м�ʯ�ҵĸ���ܵ������� ��
��4������ʢ�к�Ca(OH)2 0.02mol��ʯ��ˮ����ʵ�������й�����0.82 g���������ʵ���в��CN-�������İٷ��ʵ��� �����ò��ֵ��ʵ�ʴ����İٷ������ƫ�ͣ����Ҫ˵�����ܵ�����һ��ԭ�� ��
��5�������һ�������ȷ�ȵĽ��飨Ҫ�пɲ����ԣ�����ʹ������ù��ڸ��ӣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ������ѧ�ڵ������¿���ѧ�Ծ� ���ͣ�ʵ����
��14�֣���Ƴ������������ƹ��գ������ŷŵķ�ˮ�к��еľ綾CN�����ӣ����������ƹ�����������������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN������������CNO�����������������¼�����NaClO������N2��CO2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ����ⶨCN���������İٷ��ʡ�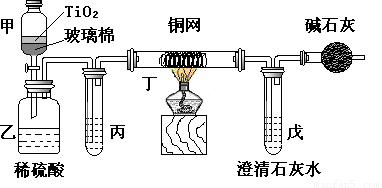
��Ũ����CN�����ӵ���ˮ�����NaClO��Һ�Ļ��Һ��200mL������CN����Ũ��Ϊ0.05mol��L��1��������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺
�����з�Ӧ�����ӷ���ʽΪ______________ ___________��
���������ɵ������N2��CO2�⣬����HCl��������Cl2�ȣ�����ʵ����ͨ���ⶨ������̼������ȷ����CN���Ĵ���Ч��������м���ij����Լ���__ __������ĸ��
a������ʳ��ˮ b������NaHCO3��Һ c��ŨNaOH��Һ d��Ũ����
�Ƕ���ʵ���е�������_____ __________________ ��
װ�м�ʯ�ҵĸ���ܵ�������___________ ___________��
������ʢ�к�Ca(OH)20.02mol��ʯ��ˮ����ʵ�������й�����0.82 g���������ʵ���в��CN���������İٷ��ʵ���___ _____����˵���ò��ֵ��ʵ�ʴ����İٷ������ƫ����ƫ��_ ___����Ҫ˵�����ܵ�ԭ��________________________ _________ _________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ƴ������������ƹ��գ������ŷŵķ�ˮ�к��еľ綾CN�����ӣ����������ƹ�����������������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN������������CNO�����������������¼�����NaClO������N2��CO2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ����ⶨCN���������İٷ��ʡ�
��Ũ����CN�����ӵ���ˮ�����NaClO��Һ�Ļ��Һ200 mL������CN����Ũ��Ϊ0.05 mol?L��1��������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ�����

������ ���з�Ӧ�����ӷ���ʽΪ_______________�����з�Ӧ�����ӷ���ʽΪ________
������ �������ɵ������N2��CO2�⣬����HCl��������Cl2�ȣ�����ʵ����ͨ���ⶨ������̼������ȷ����CN���Ĵ���Ч�������м���ij����Լ���____________������ĸ��
a������ʳ��ˮ b������NaHCO3��Һ
c��ŨNaOH��Һ d��Ũ����
����������ʵ���е�������__________________________________
����������ʢ��������ʯ��ˮ����ʵ�������й�����0.82 g���������ʵ���в��CN���������İٷ��ʵ���_________________����˵���ò��ֵ��ʵ�ʴ����İٷ������ƫ����ƫ�ͣ���Ҫ˵������______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com