
ÓŠ»śĪļ¼üĻߏ½½į¹¹µÄĢŲµćŹĒŅŌĻߏ¾¼ü£¬ĆæøöÕŪµćŗĶĻ߶Ė“¦±ķŹ¾ÓŠŅ»øöĢ¼Ō×Ó£¬²¢ŅŌĒā²¹×ćĖļü£¬C”¢H²»±ķŹ¾³öĄ“£¬ĘäĖüŌ×Ó»ņŌ×ÓĶÅŅŖ±ķŹ¾³öĄ“£¬ĄżČē£ŗCH3CHOHCH3µÄ¼üĻߏ½½į¹¹ĪŖ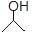 ”£ CH3CH=CHCH3ŹĒŹÆÓĶĮŃ½āµÄ²śĪļÖ®Ņ»£¬ĖüµÄ¼üĻߏ½½į¹¹æɱķŹ¾ĪŖ
”£ CH3CH=CHCH3ŹĒŹÆÓĶĮŃ½āµÄ²śĪļÖ®Ņ»£¬ĖüµÄ¼üĻߏ½½į¹¹æɱķŹ¾ĪŖ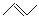 ”£
ӣ
¢ÅCH3CH=CHCH3µÄĆū³ĘĪŖ £¬ĘäĖłŗ¬¹ŁÄÜĶŵĽį¹¹Ź½ĪŖ £¬ÓėH2ŌŚŅ»¶ØĢõ¼žĻĀ·¢Éś¼Ó³É·“Ó¦£¬Ęä²śĪļµÄĶ¬·ÖŅģ¹¹ĢåµÄ¼üĻߏ½½į¹¹ĪŖ ”£
¢ĘĶź³ÉĻĀĮŠ·“Ó¦·½³ĢŹ½£¬²śĪļÓĆ¼üĻߏ½±ķŹ¾£ŗ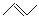 +Br2”ś £¬·“Ó¦ĄąŠĶĪŖ ·“Ó¦”£
+Br2”ś £¬·“Ó¦ĄąŠĶĪŖ ·“Ó¦”£
¢Ē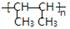 ŹĒŅ»ÖÖ¼Ó¾Ū²śĪļ£¬ŌņĘ䵄ĢåµÄ½į¹¹¼ņŹ½ĪŖ £¬ĘäĮ“½ŚĪŖ
ŹĒŅ»ÖÖ¼Ó¾Ū²śĪļ£¬ŌņĘ䵄ĢåµÄ½į¹¹¼ņŹ½ĪŖ £¬ĘäĮ“½ŚĪŖ
ӣ
¢ČŠ“³öÓėCH3CH=CHCH3ŗ¬ÓŠĻąĶ¬¹ŁÄÜĶŵÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½
”££ØČĪŠ“Ņ»ÖÖ£©

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ń°ÕŅĒå½ąÄÜŌ“Ņ»Ö±ŹĒ»Æѧ¼ŅŬĮ¦µÄ·½Ļņ£¬ĻĀĮŠ¹ŲÓŚÄÜŌ“µÄĖµ·ØÖŠ“ķĪóµÄŹĒ(””””)
A£®ĒāĘųČ¼ÉÕČČøߣ¬ĘäČ¼ÉÕ²śĪļŹĒĖ®£¬ŹĒŅ»ÖÖĄķĻėµÄĒå½ąČ¼ĮĻ
B£®ĄūÓĆĢ«ŃōÄܵČĒå½ąÄÜŌ““śĢę»ÆŹÆČ¼ĮĻ£¬ÓŠĄūÓŚ½ŚŌ¼×ŹŌ“”¢±£»¤»·¾³
C£®ĆŗµÄĘų»Æ¼¼ŹõŌŚŅ»¶Ø³Ģ¶ČÉĻŹµĻÖĮĖĆŗµÄøߊ§”¢Ēå½ąĄūÓĆ
D£®ŹÆÓĶ×÷ĪŖÖŲŅŖµÄæÉŌŁÉśÄÜŌ“Ó¦øƱ»¾”ĮæµŲĄūÓĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖI2+SO32-+H2O===SO42-+2I-+2H+”£Ä³ĪŽÉ«ČÜŅŗÖŠÖ»æÉÄÜŗ¬ÓŠI-£¬NH4+,Ba2+, SO32-,MnO4-ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ£¬ČōĻņøĆČÜŅŗÖŠµĪ¼ÓÉŁĮæµÄäåĖ®£¬ČÜŅŗČŌĪŖĪŽÉ«£¬ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ ( )
A.øĆČÜŅŗÖŠæĻ¶Ø²»ŗ¬I- B. øĆČÜŅŗÖŠæÉÄÜŗ¬ÓŠBa2+
C. øĆČÜŅŗÖŠæĻ¶Øŗ¬ÓŠNH4+ D. øĆČÜŅŗÖŠæÉÄÜŗ¬ÓŠMnO4-
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØĢõ¼žĻĀ£¬·Ö±š¶Ō·“Ó¦A(s)+B(g) 2C(g) (Õż·“Ó¦ĪüČČ)½ųŠŠČēĻĀ²Ł×÷£ØÖ»øıäøĆĢõ¼ž£©£ŗ
2C(g) (Õż·“Ó¦ĪüČČ)½ųŠŠČēĻĀ²Ł×÷£ØÖ»øıäøĆĢõ¼ž£©£ŗ
¢ŁŌö“óAµÄÓĆĮæ ¢ŚĖõŠ”ČŻĘ÷µÄČŻ»ż ¢ŪÉżøßĢåĻµĪĀ¶Č ¢Ü¼õŠ”ĢåĻµÖŠCµÄĮæ
ÉĻŹö“ėŹ©ÖŠŅ»¶ØÄÜŹ¹Õż·“Ó¦ĖŁĀŹ¼ÓæģµÄŹĒ
A£®¢Ł¢Ū B£®¢Ś¢Ū C£®¢Ł¢Ś¢Ū D£®¢Ł¢Ś¢Ū¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄūĆŹĻ©ŹĒŅ»ÖÖŹ³ÓĆĻćĮĻ£¬Ęä½į¹¹¼ņŹ½ČēĶ¼£¬ÓŠ¹ŲÄūĆŹĻ©µÄ·ÖĪö“ķĪóµÄŹĒ
A£®ŌŚŅ»¶ØĢõ¼žĻĀ£¬1molÄūĆŹĻ©æÉÓė2molH2ĶźČ«¼Ó³É
B£®ÄūĆŹĻ©µÄŅ»ĀČ“śĪļÓŠ7ÖÖ
C£®ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÄūĆŹĻ©æÉ·¢Éś¼Ó³É”¢Č”“ś”¢Ńõ»Æ”¢»¹Ō·“Ó¦
D£®ÄūĆŹĻ©·Ö×ÓÖŠĖłÓŠĢ¼Ō×Ó²»æÉÄܶ¼ŌŚĶ¬Ņ»Ę½Ćę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÕżĪóÅŠ¶Ļ£¬ÕżČ·µÄ“ņ”°”Ģ”±£¬“ķĪóµÄ“ņ”°”Į”±
(1)NH3”¢SO2µÄĖ®ČÜŅŗ¾łµ¼µē£¬ĖłŅŌNH3”¢SO2¾łŹōÓŚµē½āÖŹ(””””)
(2)Ēæµē½āÖŹ±„ŗĶČÜŅŗŅ»¶Ø±ČČõµē½āÖŹ±„ŗĶČÜŅŗµÄµ¼µēŠŌĒæ(””””)
(3)NaClČÜŅŗÄܵ¼µē£¬¹ŹNaClČÜŅŗĪŖµē½āÖŹ(””””)
(4)Fe”¢Cu”¢AgČŪ»ÆÄܵ¼µē£¬¹ŹFe”¢Cu”¢AgĪŖµē½āÖŹ(””””)
(5)H2SČÜÓŚĖ®µÄµēĄė·½³ĢŹ½ĪŖ
H2SH£«£«HS££¬HS£H£«£«S2£(””””)
(6)ĮņĖįĒāÄĘČÜÓŚĖ®µÄµēĄė·½³ĢŹ½ĪŖ
NaHSO4Na£«£«H£«£«SO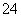 (””””)
(””””)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×ÓÄÜŌŚČÜŅŗÖŠ“óĮæ¹²“ęµÄŹĒ(””””)
A£®Na£«”¢NH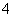 ”¢CO
”¢CO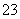 ”¢Br£
”¢Br£
B£®Fe2£«”¢H£«”¢NO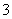 ”¢Cl£
”¢Cl£
C£®Al3£«”¢Ca2£«”¢HCO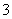 ”¢SO
ӢSO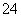
D£®Fe3£«”¢Cu2£«”¢NO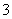 ”¢OH£
”¢OH£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŗ£Ė®µ»Æ¾ßÓŠ¹ć·ŗµÄÓ¦ÓĆĒ°¾°£¬µ»ÆĒ°Šč¶Ōŗ£Ė®½ųŠŠŌ¤“¦Ąķ”£
(1)Ķس£ÓĆĆ÷·Æ[K2SO4·Al2(SO4)3·24H2O]×÷»ģÄż¼Į£¬½µµĶ×Ē¶Č”£Ć÷·ÆĖ®½āµÄĄė×Ó·½³ĢŹ½ŹĒ____________________________________”£
(2)ÓĆĻĀĶ¼ĖłŹ¾NaClOµÄ·¢Éś×°ÖƶŌŗ£Ė®½ųŠŠĻū¶¾ŗĶĆšŌ哦Ąķ”£
¢Ł×°ÖĆÖŠÓÉNaCl×Ŗ»ÆĪŖNaClO¹ż³ĢÖŠµÄĄė×Ó·½³ĢŹ½__________________”¢__________________£¬×ܵĥė×Ó·½³ĢŹ½______________”£
¢Śŗ£Ė®ÖŠŗ¬ÓŠCa2£«”¢Mg2£«”¢HCO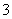 µČŌÓÖŹĄė×Ó£¬“¦Ąķ¹ż³Ģ֊װÖƵÄŅõ¼«ŅײśÉśĖ®¹ø£¬ĘäÖ÷ŅŖ³É·ÖŹĒMg(OH)2ŗĶCaCO3”£Éś³ÉCaCO3µÄĄė×Ó·½³ĢŹ½ŹĒ____________________________”£
µČŌÓÖŹĄė×Ó£¬“¦Ąķ¹ż³Ģ֊װÖƵÄŅõ¼«ŅײśÉśĖ®¹ø£¬ĘäÖ÷ŅŖ³É·ÖŹĒMg(OH)2ŗĶCaCO3”£Éś³ÉCaCO3µÄĄė×Ó·½³ĢŹ½ŹĒ____________________________”£

¢ŪČōĆæøō5”«10 minµ¹»»Ņ»“Īµē¼«µēŠŌ£¬æÉÓŠŠ§µŲ½ā¾öŅõ¼«µÄ½į¹øĪŹĢā”£
ŹŌÓƵē¼«·“Ó¦Ź½²¢½įŗĻ±ŲŅŖµÄĪÄ×Ö½ųŠŠ½āŹĶ____________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ė«ŃõĖ®(H2O2)ŗĶĖ®¶¼ŹĒ¼«Čõµē½āÖŹ£¬µ«H2O2±ČH2OøüĻŌĖįŠŌ”£
(1)Čō°ŃH2O2æ“³ÉŹĒ¶žŌŖČõĖį£¬ĒėŠ“³öŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½£ŗ
________________________________________________________________________
________________________________________________________________________ӣ
£Ø2£©¼ųÓŚH2O2ĻŌČõĖįŠŌ£¬ĖüÄÜĶ¬Ēæ¼ī×÷ÓĆŠĪ³ÉÕżŃĪ£¬ŌŚŅ»¶ØĢõ¼žĻĀŅ²æÉŠĪ³ÉĖįŹ½ŃĪ”£ĒėŠ“³öH2O2ÓėBa(OH)2×÷ÓĆŠĪ³ÉŃĪµÄ»Æѧ·½³ĢŹ½£ŗ
________________________________________________________________________
________________________________________________________________________ӣ
(3)Ė®µēĄėÉś³ÉH3O£«ŗĶOH£½Š×÷Ė®µÄ×ŌżµēĄė”£Ķ¬Ė®Ņ»Ńł£¬H2O2Ņ²ÓŠ¼«Ī¢ČõµÄ×ŌżµēĄė£¬Ęä×ŌżµēĄėµÄ·½³ĢŹ½ĪŖ_______________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com