
���� ��1���Ƚ����ʵİ��ռ�Ρ����˳����࣬�ٸ����ε�ˮ���������ʵĵ����ص�Ƚ�PH���ٰ�����Һ��PH�ɴ�С��������
��2����NaHCO3��Һ�д���̼��������ӵ�ˮ��ƽ��͵���ƽ�⣻ˮ�ĵ���ƽ�⣻��Һ�Լ�������Ϊ��Һ��̼��������ӵ�ˮ��̶ȴ��ڵ���̶ȣ�
��3�������ܶȻ���������Һ������������Ũ�ȼ����c��Cu2+������0.1mol/L����ͭ��Һ��ͨ�����H2S���壬���������Ũ��Ϊ0.1mol/L��������Һ�ĵ����Լ���H+Ũ�ȣ�
��� �⣺��1�����ڼ��Ϊ����Ba��OH��2��NaOH��Ũ����ͬʱ�����Ԣܣ��ݣ�
ˮ��ʼ��Ե�Ϊ����CH3COONa����ˮ��̶Ƚ�С����pH���ܣ��ݣ��ޣ�
��Һ�����Ե�Ϊ����KCl��
��Һ�����Ե��У��٢ڢۢ࣬���Т�ΪһԪǿ�ᣬ��Ϊ��Ԫǿ�ᣬ��Ϊ���ᣬ��ˮ������ԣ�Ũ����ͬʱ��
��Һ��pH���ڣ��٣��ۣ��࣬
�ۺ����Ϸ�����֪����Һ��pH��С�����˳����Ϊ���ڢ٢ۢ�ߢޢݢܣ�
�ʴ�Ϊ���ڢ٢ۢ�ߢޢݢܣ�
��2����NaHCO3��Һ�д���̼��������ӵ�ˮ��ƽ��Ϊ��HCO3+H2O?H2CO3+OH-������ƽ��Ϊ��HCO3-?H++CO32-������ˮ��Ϊ������̼��������ӵ�ˮ��̶ȴ��ڵ���̶���Һ�����Լ��ԣ�
�ʴ�Ϊ��HCO3+H2O?H2CO3+OH-��
��3���������Cu��OH��2���ܶȻ�����ȷ��pH=8ʱ��c��OH-��=10-6mol/L��Ksp[Cu��OH��2]=2.2��10-20����c��Cu2+��=$\frac{2.2��1{0}^{-20}}{��{1��1{0}^{-6}��}^{2}}$=2.2��10-8mol•L-1��
��0.1mol•L-1����ͭ��Һ��ͨ�����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�����Ϊ���ᣬc��SO42-�����䣬Ϊ0.1mol•L-1���ɵ���غ��֪c��H+��Ϊ0.2mol•L-1��
�ʴ�Ϊ��2.2��10-8mol•L-1��0.2mol•L-1��
���� ���⿼������Ӧ�Ķ����жϡ���������ܽ�ƽ�⼰������֪ʶ����Ŀ�Ѷ��еȣ�ע��������Һ���������ҺpH�ļ��㷽������ȷ��������ܽ�ƽ�⼰Ӧ�÷���������������ѧ���ķ�����������������ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���ữѧʽ | HF | HClO | H2CO3 |
| ����ƽ�ⳣ�� | 6.8��10-9 | 4.7��10-15 | K1=4.4��10-7 K2=4.7��10-11 |
| A�� | �����£�ͬ���ʵ���Ũ�ȵ�HF��H2CO3��Һ��ǰ�ߵ�c��H+����С | |
| B�� | ��ij��Һ��c��F-��=c��ClO-����������Һ�е���HCl��F-��ClO-�����H+ | |
| C�� | ������Na2CO3��Һ����μ���ϡ������������������Һ��c��HCO3-����������С | |
| D�� | ͬ���£����������c��H+����HF��HClO�ֱ���NaOH��ȫ��Ӧ�����ĵ�����NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3$\stackrel{O_{2}����}{��}$NO$\stackrel{O_{1}��H_{2}O}{��}$HNO3 | |
| B�� | MnO2$��_{��}^{Ũ����}$Cl2$\stackrel{ʯ��ˮ}{��}$Ư�� | |
| C�� | ��ȡMg����ˮ$\stackrel{Ca��OH��_{2}}{��}$Mg��OH��2$\stackrel{����}{��}$MgO$\stackrel{���}{��}$Mg | |
| D�� | ������$��_{����}^{����¯}$SO2$��_{O_{2}}^{�Ӵ���}$SO3$��_{ˮ}^{������}$ϡH2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������̼ͨ������������������Һ��CO2+2OH-�TCO32-+H2O | |
| B�� | ̼��������ᷴӦ��CO32-+2H+�TH2O+CO2�� | |
| C�� | ���������Ʒ�����ˮ�У�Na+H2O�TNa++OH-+H2�� | |
| D�� | ̼�������Һ�м�����������������Һ��Ca2++HCO3-+OH-�TCaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
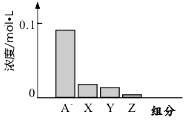 �����£�0.2mol/L��һԪ��HA���Ũ�ȵ�NaOH��Һ�������Ϻ�������Һ�ж�������ּ�Ũ����ͼ��ʾ������˵����ȷ���ǣ�������
�����£�0.2mol/L��һԪ��HA���Ũ�ȵ�NaOH��Һ�������Ϻ�������Һ�ж�������ּ�Ũ����ͼ��ʾ������˵����ȷ���ǣ�������| A�� | HAΪǿ�� | B�� | �û��ҺpH=7 | ||
| C�� | ͼ��X��ʾHA��Y��ʾOH-��Z��ʾH+ | D�� | �û����Һ�У�c��A-��+c��Y��=c��Na+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com