ͭ��Ũ���ᷴӦ��ʵ��װ����ͼ1��ʾ��
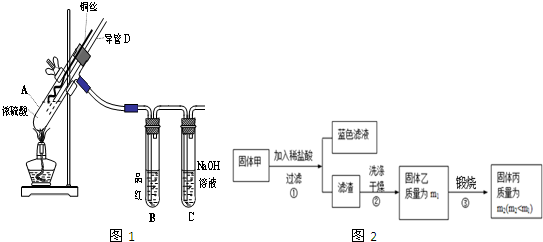
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ��
��
��Ӧ�������Թ�B�е�������
���Թ�C��������
��
��2������D���¶ˣ����߶Σ�Ӧλ��
��Һ���ϡ�Һ���£�������D�������У���ʵ��������ų�װ���е�SO
2����
��
ʵ���з����Թ��ڳ��˲�����ɫ�����⣬��ͭ˿���滹�к�ɫ��������ɣ����п��ܺ���CuO��Cu
2O��CuS��Cu
2S��Ϊ̽���ijɷ֣�������ͼ2��ʵ�飮
�������Ͽ�֪��Cu
2O+2HCl�TCuCl
2+Cu+H
2O��2Cu
2O+O
2 4CuO��2CuS+3O
22CuO+2SO
2��Cu
2S+2O
22CuO+SO
2��CuS��Cu
2S��ϡHCl����Ӧ��
��3���������ڿ���������ʱ��ʹ�õ�ʵ���������˲����������żܡ��ƾ����⣬�������У�
��
��4�����չ����У���������Ӧ�⣬�����ܷ�����Ӧ�ķ���ʽΪ
��
��5�����ۣ�������CuO�����϶����е�������
��










 ����һ�����ϣ�һ�ֺϳ�·�����£�
����һ�����ϣ�һ�ֺϳ�·�����£� ��
��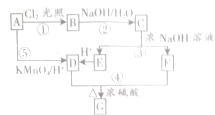
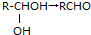 +H2O������C�ܷ���������Ӧ������C��Ũ�������¿��Է�������������ԭ��Ӧ����E��F����E��F������̼ԭ�ӵ���Ŀ��ͬ��
+H2O������C�ܷ���������Ӧ������C��Ũ�������¿��Է�������������ԭ��Ӧ����E��F����E��F������̼ԭ�ӵ���Ŀ��ͬ��