
����Ŀ�������仯�����ڹ�ũҵ�������ճ�����������Ҫ��;����ش��������⣺
��1��Alԭ�ӵļ۵����Ų�ͼΪ_________________________________��Na��Mg��Al�ĵ�һ��������С�����˳��Ϊ________________________��
��2��ij������Ԫ�ص����Ļ�ѧʽΪBe3Al2(Si6O18)������Siԭ�ӵ��ӻ��������Ϊ________��
��3����ҵ������������������̼�����ڸ��������¿��Ʊ�һ��������ṹ��Ԫ�ĸ��½ṹ�մɣ��侧����ͼ��ʾ��
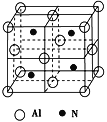
�ٸ��Ʊ���Ӧ�Ļ�ѧ����ʽΪ___________________________________.
�ڸû�����ľ�������Ϊ_______________���þ�������____����ԭ�ӣ��þ����ı߳�Ϊa pm����þ������ܶ�Ϊ____________g��cm��3��
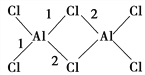
��4��AlCl3����Է�������Ϊ133.5��183 ����ʼ������������ˮ�����ѵȣ��������(Al2Cl6)�Ľṹ��ͼ��ʾ��ͼ��1������Ϊ206 pm,2������Ϊ221 pm���Ӽ����γɽǶȷ���1����2��������__________________________________________��
��5��LiAlH4��һ������Ļ�ԭ�����ɽ�����ֱ�ӻ�ԭ�ɴ���
CH3COOH ![]() CH3CH2OH
CH3CH2OH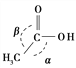
CH3COOH��������������������Ŀ֮��Ϊ________�������м�����________������(������������������������С����)��
���𰸡� 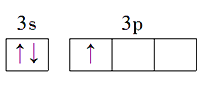 Na<Al<Mg sp3 Al2O3��N2��3C
Na<Al<Mg sp3 Al2O3��N2��3C![]() 3CO��2AlN ԭ�Ӿ��� 4
3CO��2AlN ԭ�Ӿ��� 4 ![]() 1��Ϊ��ԭ�ӡ���ԭ�Ӹ��ṩһ�������γɵĹ��ۼ���2��Ϊ��ԭ���ṩ�µ��Ӷԡ���ԭ���ṩ�չ���γɵ���λ�� 1��7 С��
1��Ϊ��ԭ�ӡ���ԭ�Ӹ��ṩһ�������γɵĹ��ۼ���2��Ϊ��ԭ���ṩ�µ��Ӷԡ���ԭ���ṩ�չ���γɵ���λ�� 1��7 С��
����������1������۵����Ų�ͼ����һ�����ܵĹ��ɣ�Al��������Ԫ�أ��۵���ָ�������������������Al�ļ۵����Ų�ͼΪ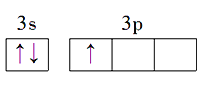 ��ͬ���ڴ������ҵ�һ����������IIA>IIIA��VA>VIA���������Ԫ�صĵ�һ�����ܵ�˳����Na<Al<Mg����2�������ӻ�������жϣ���д�����������ʽ3BeO��Al2O3��6SiO2��1molSiO2�к���4mol�Ҽ����µ��Ӷԣ����Si���ӻ�����Ϊsp3����3�����黯ѧ��Ӧ����ʽ����д�����������жϡ������ļ��㣬�ٸ��ݾ����Ľṹ��Al�ڶ�������ģ�Al�ĸ���Ϊ8��1/8��6��1/2=4��Nλ�ھ����ڲ�������Ϊ4����ѧʽΪAlN��Al2O3��N2��3C
��ͬ���ڴ������ҵ�һ����������IIA>IIIA��VA>VIA���������Ԫ�صĵ�һ�����ܵ�˳����Na<Al<Mg����2�������ӻ�������жϣ���д�����������ʽ3BeO��Al2O3��6SiO2��1molSiO2�к���4mol�Ҽ����µ��Ӷԣ����Si���ӻ�����Ϊsp3����3�����黯ѧ��Ӧ����ʽ����д�����������жϡ������ļ��㣬�ٸ��ݾ����Ľṹ��Al�ڶ�������ģ�Al�ĸ���Ϊ8��1/8��6��1/2=4��Nλ�ھ����ڲ�������Ϊ4����ѧʽΪAlN��Al2O3��N2��3C![]() 2AlN��3CO����AlN�������Ǹ��½ṹ���մɣ�˵��AlN���۷е�ߣ���AlN����ԭ�Ӿ��壻����������������������4����ԭ�ӣ����������Ϊ(a��10��10)3cm3������������Ϊ
2AlN��3CO����AlN�������Ǹ��½ṹ���մɣ�˵��AlN���۷е�ߣ���AlN����ԭ�Ӿ��壻����������������������4����ԭ�ӣ����������Ϊ(a��10��10)3cm3������������Ϊ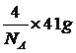 �������ܶȵĶ��壬�������ܶ�Ϊ
�������ܶȵĶ��壬�������ܶ�Ϊ![]() g/cm3����4��������λ����������ĿAlCl3�����ʣ��Ƴ�AlCl3���ڷ��Ӿ��壬1��Alԭ���ṩ�������ӣ���������ԭ���γɹ��ۼ�������Al2Cl6�Ľṹ��˵��Al����λ������2Ϊ��λ�������������1��Ϊ��ԭ�ӡ���ԭ�Ӹ��ṩһ�������γɵĹ��ۼ���2��Ϊ��ԭ���ṩ�µ��Ӷԡ���ԭ���ṩ�չ���γɵ���λ������5�����黯ѧ����Ŀ���жϣ��ɼ�ԭ��֮��ֻ���γ�һ���Ҽ������1mol�����к��ЦҼ����ʵ���Ϊ7mol���Ȼ��к���̼��˫�������1mol�����к��Цм������ʵ���Ϊ1mol������������������Ŀ֮��Ϊ1��7���ʻ��ϵ�Oԭ���������µ��Ӷԣ��µ��Ӷ�֮��ij������ڳɼ����Ӷ�֮��ij�������˷����м�����С����������
g/cm3����4��������λ����������ĿAlCl3�����ʣ��Ƴ�AlCl3���ڷ��Ӿ��壬1��Alԭ���ṩ�������ӣ���������ԭ���γɹ��ۼ�������Al2Cl6�Ľṹ��˵��Al����λ������2Ϊ��λ�������������1��Ϊ��ԭ�ӡ���ԭ�Ӹ��ṩһ�������γɵĹ��ۼ���2��Ϊ��ԭ���ṩ�µ��Ӷԡ���ԭ���ṩ�չ���γɵ���λ������5�����黯ѧ����Ŀ���жϣ��ɼ�ԭ��֮��ֻ���γ�һ���Ҽ������1mol�����к��ЦҼ����ʵ���Ϊ7mol���Ȼ��к���̼��˫�������1mol�����к��Цм������ʵ���Ϊ1mol������������������Ŀ֮��Ϊ1��7���ʻ��ϵ�Oԭ���������µ��Ӷԣ��µ��Ӷ�֮��ij������ڳɼ����Ӷ�֮��ij�������˷����м�����С����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(G)���з��ɻ�����ζ,�����ڵ����ζʳƷ�㾫�����û�ױƷ�㾫��һ���÷�����A�ϳ�F�Ĺ���·������:
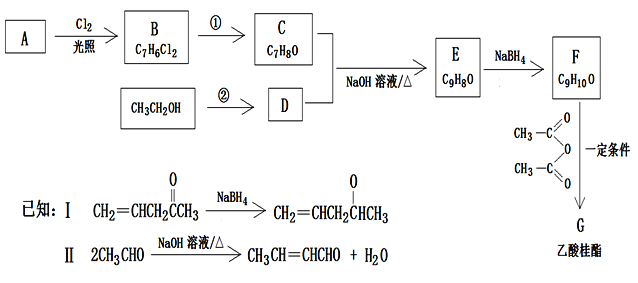
�ش���������:
��1��E�ṹ��ʽΪ____________��C��������____________
��2���ٵġ���Ӧ������Ϊ________;�ڵķ�Ӧ����Ϊ__________.
��3��F�����������ŵ�����Ϊ___________
��4��д��F![]() G��Ӧ�Ļ�ѧ����ʽ___________
G��Ӧ�Ļ�ѧ����ʽ___________
��5��F��ͬ���칹����,ͬʱ����������������__��(�����������칹);������FeCl3��Һ������ɫ���ڱ�����������ȡ��������һ��̼̼˫�������к˴Ź���������5���,�ҷ����֮��Ϊ1:2:2:2:3�Ľṹ��ʽΪ__________.
��6���۲챾��ϳ�·�ߵ��Լ�������,�������Ϻϳ�·���е������Ϣ����д����HCHO��CH3CHO���Ʊ�CH2=CHCH2OOCCH3�ĺϳ�·��ͼ��(��Ӧ�P����д�ɽṹ��ʽ)________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͨ��ij��A����Է�������Ϊ28������ʹ������Ȼ�̼��Һ��ɫ��F�߷��ӻ������������ת����ϵ��
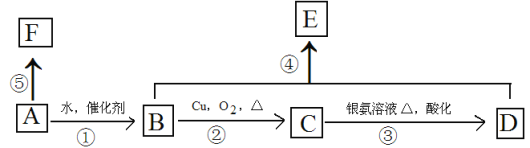
��Ҫ����գ�
��1��д��C�����������ŵ����� ��ָ���ܵķ�Ӧ���� ��
��2����Bת��ΪA�����跴Ӧ����Ϊ�� ��
��3��д��B��F�ṹ��ʽ��B ��F ��
��4��д����Ӧ�۶�Ӧ�Ļ�ѧ����ʽ�� ��
��5��д����Ӧ�ܶ�Ӧ�Ļ�ѧ����ʽ�� ��
��6��E��ͬ���칹���ж��֣���������NaHCO3��Ӧ��ͬ���칹�干�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Z�����·�Ӧ�õ���C4H9Br![]() Y
Y![]() Z��Z�Ľṹ��ʽ�������ǣ� ��
Z��Z�Ľṹ��ʽ�������ǣ� ��
A��CH3CH2CHBrCH2Br
B��CH3CH��CH2Br��2
C��CH3CHBrCHBrCH3
D��CH2BrCBr��CH3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.2 mol MnO2��50 mL 12 mol/L�����Ϻ����ȣ���Ӧ��ȫ�������µ���Һ�м�������AgNO3��Һ������AgCl�������ʵ���Ϊ(����������Ļӷ�)
A. ����0.3 mol B. ��0.3 mol
C. ����0.3 mol D. ���Ͻ��۶�����ȷ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������һ�����Ƹ�Ѫѹ��ҩ����м��壬����ͨ�����·����ϳɣ�
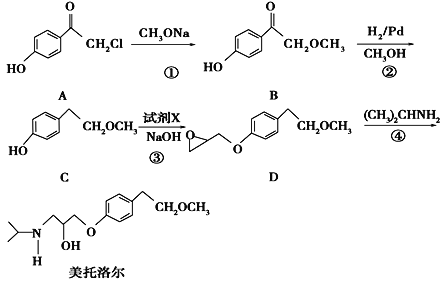
��ش��������⣺
��1��д��C�еĹ����ŵ�����Ϊ_________________.
��2����������ķ���ʽ________________.
��3��д����Ӧ�ٵĻ�ѧ����ʽ__________________________________________����Ӧ�ڵķ�Ӧ������_____________
��4����Ӧ���м�����Լ�X�ķ���ʽΪC3H5OCl��X�Ľṹ��ʽΪ____________________.
��5����������������B��ͬ���칹������_______�֣����к˴Ź������������ֲ�ͬ��ѧ�������⣬�ҷ������Ϊ3��2��2��1��1��1����________________________(д�ṹ��ʽ)
���ܷ���������Ӧ�����ܷ���ˮ��
������FeCl3��Һ������ɫ��Ӧ
��ֻ��һ����
��6����������֪ʶ�������Ŀ���������Ϣ��д����![]() ��
��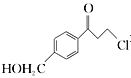 Ϊԭ���Ʊ�
Ϊԭ���Ʊ�![]() �ĺϳ�·������ͼ(���Լ���ѡ)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(���Լ���ѡ)���ϳ�·������ͼʾ�����£�![]() _______________________
_______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��ݸ�����ͼ�������жϣ�����ȷ����
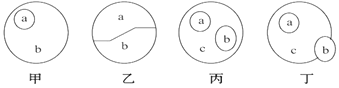
A. ��ͼ��a��b���Ա�ʾ���Ǻ͵��ǵĹ�ϵ
B. ������a��b�ֱ����DNA��RNA������ͼ���Դ���ԭ��ϸ���ڵĺ���
C. ��ͼ��a��b��c�ɷքe��ʾ��֬���̴���֬��֮��Ĺ�ϵ
D. ��ͼa��b��c�ɷֱ��ʾ���ء�ø�͵�����֮��Ĺ�ϵ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����CH3CH2CH2Br�Ʊ�CH3CH(OH)CH2OH�����η����ķ�Ӧ���ͺͷ�Ӧ��������ȷ����
ѡ�� | ��Ӧ���� | ��Ӧ���� |
A | �ӳɡ�ȡ������ȥ | KOH����Һ/���ȡ�KOHˮ��Һ/���ȡ����� |
B | ��ȥ���ӳɡ�ȡ�� | NaOH����Һ/���ȡ����¡�KOHˮ��Һ/���� |
C | ������ȡ������ȥ | ���ȡ�KOH����Һ/���ȡ�KOHˮ��Һ/���� |
D | ��ȥ���ӳɡ�ˮ�� | NaOHˮ��Һ/���ȡ����¡�NaOH����Һ/���� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com