
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100��(���֮��)
��ʹ�á���ش��������⣺

��1���á�84����Һ�������ʵ���Ũ��ԼΪ_____mol��L��1��
��2��ȡ����������ĸ�����Һʱ�������������л�����ȡ����Ķ��ٶ��仯����________(����ĸ)��
A����Һ��NaClO�����ʵ��� B����Һ��Ũ��
C����Һ��NaClO��Ħ������ D����Һ���ܶ�
��3����ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ���ش��������⡣
����ͼ��ʾ�������У���Щ�Dz���Ҫ������������Һ����Ҫ_____________��������

����Ҫ����NaClO���������Ϊ_______ g
��4����84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������200 mL 2.3 mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ________ mol��L��1��
������Ũ��������Ϊ________ mL��
���������Ƶ�ϡ����Ũ��ƫС�������п��ܵ�ԭ���������ȷ����_______��
A������ǰ������ƿ������������ˮ B����ȡŨ����ʱ������Һ��İ�Һ��
C��δ��ȴ������ת��������ƿ���� D������ʱ��������Һ�İ�Һ��
���𰸡�4.0A����������ͷ�ι�149.04.625.0D
��������
��1������c=![]() =1000��1.19��25%/74.5mol��L��1��4.0mol��L��1����2��A�����ʵ����ʵ���Ũ�Ȳ��䣬����c=n/V����������ͬ����ȡ��NaClO�����ʵ�����ͬ����A�������⣻B����ҺΪ��һ���������Һ��Ũ�ȱ��ֲ��䣬��B���������⣻C��NaClO��Ħ����������74.5g��mol��1����C���������⣻D���ܶȲ�������ı仯���仯����D���������⣻��3�����ù�������һ�����ʵ���Ũ�ȣ������Ҫ��������������ƽ��ҩ�ס��ձ�����������500mL ����ƿ����ͷ�ιܣ���Ҫ�IJ����������в������ͽ�ͷ�ιܣ�����Ϊʵ����û��480mL������ƿ�������Ҫ500mL������ƿ�������Ҫ������NaClO������Ϊ500��10��3��4��74.5g=149.0g����4���������Ƶ�������c(H��)=2c(H2SO4)=2��4.3mol��L��1=4.6mol��L��1����Ũ��������ʵ���Ũ��Ϊ1000��98%��1.84/98mol��L��1=18.4mol��L��1��ϡ��ǰ���������ʵ������䣬200��10��3��2.3=V(H2SO4)��10��3��18.4��V(H2SO4)=25.0mL����A������ƿ���Ƿ���ˮ����ʵ������Ӱ�죬��A���������⣻B����ȡŨ����ʱ�����Ӱ�Һ�棬������Һ�����ʵ����ʵ�������Ũ��ƫ�ߣ���B���������⣻C��δ��ȴֱ��ת�Ƶ�����ƿ��Ȼ���ݣ���ȴ�ָ������£�����ƿ����Һ��������٣�������ҺŨ��ƫ�ߣ���C���������⣻D������ʱ�����Ӱ�Һ�棬����ƿ����Һ�����ƫ�ߣ�Ũ��ƫ�ͣ���D�������⡣
=1000��1.19��25%/74.5mol��L��1��4.0mol��L��1����2��A�����ʵ����ʵ���Ũ�Ȳ��䣬����c=n/V����������ͬ����ȡ��NaClO�����ʵ�����ͬ����A�������⣻B����ҺΪ��һ���������Һ��Ũ�ȱ��ֲ��䣬��B���������⣻C��NaClO��Ħ����������74.5g��mol��1����C���������⣻D���ܶȲ�������ı仯���仯����D���������⣻��3�����ù�������һ�����ʵ���Ũ�ȣ������Ҫ��������������ƽ��ҩ�ס��ձ�����������500mL ����ƿ����ͷ�ιܣ���Ҫ�IJ����������в������ͽ�ͷ�ιܣ�����Ϊʵ����û��480mL������ƿ�������Ҫ500mL������ƿ�������Ҫ������NaClO������Ϊ500��10��3��4��74.5g=149.0g����4���������Ƶ�������c(H��)=2c(H2SO4)=2��4.3mol��L��1=4.6mol��L��1����Ũ��������ʵ���Ũ��Ϊ1000��98%��1.84/98mol��L��1=18.4mol��L��1��ϡ��ǰ���������ʵ������䣬200��10��3��2.3=V(H2SO4)��10��3��18.4��V(H2SO4)=25.0mL����A������ƿ���Ƿ���ˮ����ʵ������Ӱ�죬��A���������⣻B����ȡŨ����ʱ�����Ӱ�Һ�棬������Һ�����ʵ����ʵ�������Ũ��ƫ�ߣ���B���������⣻C��δ��ȴֱ��ת�Ƶ�����ƿ��Ȼ���ݣ���ȴ�ָ������£�����ƿ����Һ��������٣�������ҺŨ��ƫ�ߣ���C���������⣻D������ʱ�����Ӱ�Һ�棬����ƿ����Һ�����ƫ�ߣ�Ũ��ƫ�ͣ���D�������⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼ1��ʾ�ĵ�ѭ������̬ϵͳ����ѭ������Ҫ��ɲ��֣������Ӿ��˵�ѭ���е�����ת����
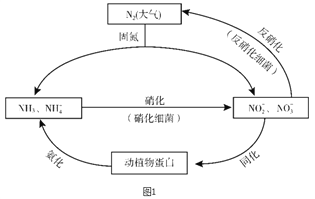
��1�������ͼ�ж�����˵����ȷ����________������ĸ��ţ���
A. �̵������У�N2ֻ��������
B. ������ϸ�������·���������������Ҫ������������
C. �����������������ֲ��˹��̵��Ե�ѭ����ɵ�Ӱ��
D. ͬ�������������У���Ԫ�ؾ�������ת�����л���
��2�����������У�NH3ת����HNO2�ķ�Ӧ�Ļ�ѧ����ʽΪ_______��
��3�������������У�CH3OH����Ϊ��Ӧ�Ļ�ԭ�����뽫�÷�Ӧ�����ӷ���ʽ����������5CH3OH + 6NO3- ![]() N2�� + 4HCO3- +��______+��
N2�� + 4HCO3- +��______+��
��4�������±����ݽ��й��㣬д����ҵ�ϳɰ���Ӧ���Ȼ�ѧ����ʽ��_______��
���ۼ� | N��N | H��H | N��H |
�Ͽ�1mol���ۼ�����������kJ�� | 946 | 436 | 391 |
��5����ⷨ�ϳɰ�����ԭ��ת���ʴ������ߣ��������洫ͳ�Ĺ�ҵ�ϳɰ����ա���ⷨ�ϳɰ�������ԭ����װ����ͼ2��ͼ3��ʾ��
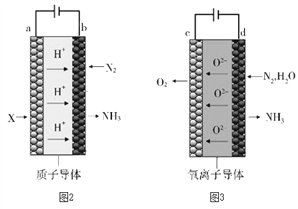
��ͼ2�У�a�缫��ͨ���XΪ_______��
��ͼ3�У�d�缫�ϵĵ缫��ӦʽΪ_______��
����ͼ2��ͼ3װ�õ�ͨ��ʱ����ͬ������ǿ����ȣ����Ч�ʷֱ�Ϊ80%��60%��������װ���в������������ʵ���֮��Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������460mL0.1mol/LNaOH��Һ���ش��������⣺
��1����ɱ�ʵ��������������У�������ƽ��ҩ�ס��ձ�������������Ͳ��_____��_____�ȡ�
��2��Ӧ��������ƽ��ȡNaOH���������Ϊ_________��
��3����������������NaOH��ҺŨ��ƫ�ߵ���_________________
A .�������õ�NaOH�������С�ձ����ܽ⣬δ����ȴ����ת�Ƶ�����ƿ�в�����
B .ҡ�Ⱥ���Һ����ڿ̶��ߣ��μ�����ˮ���̶�����ҡ��
C .����ʱ��������ƿ�Ŀ̶���
D������ƿ�ڱڸ���ˮ���δ���ﴦ��
E�� NaOH��������ձ��г���ʱ�����
��4��ȡ���������Ϊ150mL�� A��B ����NaOH ��Һ���ֱ�ͨ��һ������ CO2 ������������Һ�еμ�һ�����ʵ���Ũ�ȵ����� , ���� CO2 �����(��״��)����������������ϵ��ͼ��ʾ��
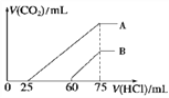
��B ���߱�����ԭ��Һͨ��CO2��������Һ�����ʵĻ�ѧʽΪ______________��
��A ���߱����������Ũ��Ϊ______mol/L��ͨ���CO2�ڱ�״���µ����Ϊ______mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д����ͼ��������������������ƣ�
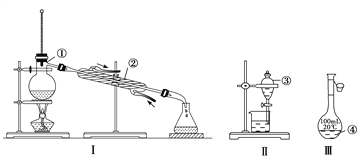
��___________����___________�� ��___________�� ��___________��
��2�������������У�ʹ��ʱ�������Ƿ�©ˮ����__________����������ţ���
��3����ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������480 mL 1 mol��L��l��ϡ���ᡣ

�ɹ�ѡ�õ������У� ����ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
������ϡ����ʱ����ȱ�ٵ�������__________��__________��д�������ƣ���
�������㣬����480 mL l mol��L��l��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ________mL������ȡ�õ�Ũ���Ỻ��ע�뵽ʢˮ���ձ�������Ͳ�ﻹ������Ũ���ᣬ���������ˮϴ�Ӻ�һ����ϴ��Һע���ձ�����ʹ���Ũ��______������ƫ������ƫ����������Ӱ��������
����ת������ƿǰ�ձ���Һ��Ӧ_______�������ʹ���Ũ��____������ƫ������ƫ����������Ӱ��������
������ʱ����ʹ��Һ�İ�Һ����̶�����ƽ�������ӻ�ʹŨ��______��������ƫ������ƫ����������Ӱ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵���������
A. ���³�ѹ�£�48gO2���е���ԭ����Ϊ3NA
B. 1.7g NH3���е�������ΪNA
C. ��״���£�11.2L�����������Ļ�������еķ�����Ϊ0.5NA
D. 1L 0.1 mol/LCH3CH2OHˮ��Һ�к�Hԭ����ĿΪ0.6NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʯ�Ͳ�Ʒ�г�����H2S�⣬�����и�����̬���л�����COS��CH3SH���ش���������:
��1��CH3SH(����)�ĵ���ʽΪ________��
��2��һ��������Ϊ:���K2CO3������˹����
��K2CO3��Һ����H2S�ķ�ӦΪK2CO3+H2S=KHS+KHCO3���÷�Ӧ��ƽ�ⳣ���Ķ���ֵΪlgK=_____(��֪:H2CO3 lgK1=-6.4��lgK2=-10.3��H2SlgK1=-7��lgK2=-19)��
����֪�����Ȼ�ѧ����ʽ:
a.2H2S(g)+3O2(g)=2SO2(g)+ 2H2O(l) ��H1=-1172kJ/mol
b.2H2S(g)+O2(g)=2S(s)+2H2O(l) ��H2=-632kJ/mol
����˹��������ķ�ӦΪSO2��H2S���巴Ӧ����S(s)����÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
��3��Dalleska�����о�������ǿ����Һ�п���H2O2����COS�����ѳ���Ӧ�Ļ�ѧ����ʽΪ______________��
��4��COSˮ�ⷴӦΪCOS(g)+H2O(g)![]() CO2(g)+H2S(g) ��H=-35.5kJ/mol��
CO2(g)+H2S(g) ��H=-35.5kJ/mol��
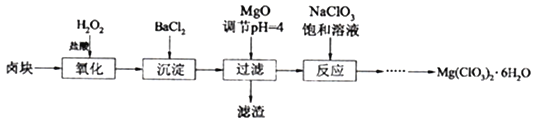
�û�����-Al2O3����������������ͬʱ���ı䷴Ӧ�¶ȣ����COSˮ��ת������ͼ1��ʾ��ij�¶�ʱ���ں����ܱ�������Ͷ��0.3molH2O(g)��0.1molCOS��COS��ƽ��ת������ͼ2��ʾ��
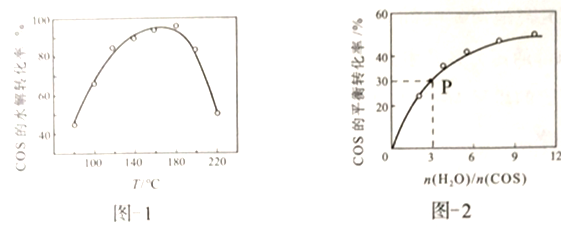
��ͼ1������-Al2O3��ˮ�⣬���¶�����ת������������ּ�С�Ŀ���ԭ����________��
����ͼ2��֪��P��ʱƽ�ⳣ��ΪK=______(������)��
��������-Al2O3��ˮ�⣬Ϊ���COS��ת���ʿɲ�ȡ�Ĵ�ʩ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���ʵ���ɫ��Ӧ�ʻ�ɫ�������ж���ȷ���ǣ� ��
A.������һ���ǽ�����B.������һ��������
C.������һ��������Ԫ��D.������һ�������м�Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�У�ʲô����Ӱ���˻�ѧ��Ӧ�����ʣ�
��1��ʳƷ����ù�䣬����Ͳ���������������
��2������طų����ݺ��������������������̺ܿ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������þ[Mg(ClO3)2]����������������ݼ��ȣ���ҵ���ģ����ǰ��ʵ�����Ȱ��������̽���ģ���Ʊ�����Mg(ClO3)2��6H2O��
��֪����±����Ҫ�ɷ�ΪMgCl2��6H2O,����MgSO4��FeCl2�����ʡ������ֻ�������ܽ��(S)���¶�(T)�仯��������ͼ��
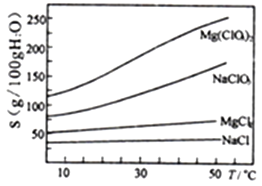
��1��±���м�H2O2��Ŀ����________________��д���÷�Ӧ�����ӷ���ʽ__________________��
��2������BaCl2��Ŀ���dz�ȥSO42-,��μ���SO42-�ѳ�����ȫ��_________________��
��3�������£���MgO����pH=4����Һ��c(Fe3+)=___________(��֪Ksp[Fe(OH)3=4��10-38]������������������Ҫ�ɷ���______________��
��4������NaClO3������Һ����NaCl����������д���÷�Ӧ�Ļ�ѧ����ʽ��___________�������ø÷�Ӧ�������ͼ����ȡMg(ClO3)2��6H2O��ʵ�鲽������Ϊ��
��ȡ��������NaClO3������Һ��ַ�Ӧ���������ᾧ����___________������ȴ�ᾧ����������ϴ�ӣ����Mg(ClO3)2��6H2O���塣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com