
�Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
��1������Ϊ���ı��洦����һ�ַ�����

�� ��ϴ��Ŀ���dz�ȥ���ı������Ȼ����Ĥ����ϴʱ��������ð����ԭ����____�������ӷ���ʽ��ʾ����Ϊ����ϴ��Һ�����Գ�����ʽ���գ�������Һ�м��������Լ��е�____����
a��NH3 b��CO2 c��NaOH d��HNO3
�� ������Ϊ��������H2SO4 ��Һ�е�⣬���ı����γ�����Ĥ�������缫��ӦΪ____��ȡ�����ϵ��Һ������NaHCO3��Һ��������ݺͰ�ɫ�����������������ԭ�������ӷ���ʽ��ʾ��_____��
��2����ͭ�ɷ�ֹ����Ʒ��ʴ�����ʱ��ͭ������ʯī���������ϵ�ԭ����______��
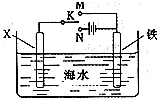
��3��������ͼװ�ã�����ģ�����ĵ绯ѧ������

��XΪ̼����Ϊ�������ĸ�ʴ������KӦ����______����
��XΪп������K����M�����õ绯ѧ��������Ϊ_______��
 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||

| 3b-a |
| 18 |
| 2a-3b |
| 36 |
| 3b-a |
| 18 |
| 2a-3b |
| 36 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���������ִ��������ϵ����ǣ�
���������ִ��������ϵ����ǣ�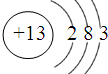

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������Ӧ�ù㷺�Ľ���֮һ���ڹ�ũҵ���������Ź㷺��Ӧ�ã��Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
��������Ӧ�ù㷺�Ľ���֮һ���ڹ�ũҵ���������Ź㷺��Ӧ�ã��Խ�����Ʒ���п���ʴ���������ӳ���ʹ���������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com