

���� Al��OH��3��H2SO4������ӦΪ2Al��OH��3+3H2SO4=Al2��SO4��3+3H2O��
��ҵ��NH4��2SO4����FeSO4������ˮ��Ȼ������Һ�м���˫��ˮ��˫��ˮ���������ԡ������������л�ԭ�ԣ����߷���������ԭ��Ӧ���������Ӻ�ˮ�����ӷ���ʽΪ2Fe2++H2O2+2H+�T2Fe3++2H2O�����õ�����Һ�м��백ˮ��������Һ��pH��ʹ������ת��ΪFe��OH��3������Ȼ����˵õ�����������Һ������������Һ�У�ͨ������õ�����NH4Al��SO4��2•12H2O�����ȹ���NH4Al��SO4��2•12H2O�������ʷֽ�����M�ͺ������ʣ�
��1��������к��������������������ӱ�˫��ˮ�����������ӣ�
��2������NH4++H2O?NH3•H2O+H+��K=$\frac{c��H{\;}^{+}��•c��N{H}_{3}•H{\;}_{2}O��}{c��NH{\;}_{4}{\;}^{+}��}$=$\frac{c��O{H}^{-}��•c��H{\;}^{+}��•c��NH{\;}_{3}•H{\;}_{2}O��}{c��NH{\;}_{4}{\;}^{+}��•c��O{H}^{-}��}$=$\frac{K{\;}_{W}}{K{\;}_{b}��NH{\;}_{3}•H{\;}_{2}O��}$���ݴ˽��
��3��NH4Al��SO4��2��Һ�����NaOH��Һ��Ӧ���ɰ�����ƫ�����ƣ�
��4��633��ʱʣ��������������Ƿ�Ӧǰ��37.75%������������茶���ֽ�����������ó���ѧʽ��
��5��M����Ҫ�ɷֵĻ�ѧʽ��NH3��H2O��SO3 ��������Ũ����������������泥��Ա��ظ����ã�
��� �⣺��1���������ӱ�����˫��ˮ�������ӣ����ݻ��ϼ����������ƽ����ʽ�����ӷ���ʽ�ǣ�2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ��ǣ�2Fe2++H2O2+2H+=2Fe3++2H2O��
��2������NH4++H2O?NH3•H2O+H+��K=$\frac{c��H{\;}^{+}��•c��N{H}_{3}•H{\;}_{2}O��}{c��NH{\;}_{4}{\;}^{+}��}$=$\frac{c��O{H}^{-}��•c��H{\;}^{+}��•c��NH{\;}_{3}•H{\;}_{2}O��}{c��NH{\;}_{4}{\;}^{+}��•c��O{H}^{-}��}$=$\frac{K{\;}_{W}}{K{\;}_{b}��NH{\;}_{3}•H{\;}_{2}O��}$=$\frac{1.0��10{\;}^{-14}}{1.75��10{\;}^{-5}}$=5.7��10-10��
�ʴ�Ϊ��5.7��10-10��
��3��NH4Al��SO4��2��Һ�����NaOH��Һ��ϼ��ȣ���Ӧ�Ļ�ѧ����ʽΪ��NH4Al��SO4��2+5NaOH $\frac{\underline{\;\;��\;\;}}{\;}$NH3��+3H2O+NaAlO2+2Na2SO4��
�ʴ��ǣ�NH4Al��SO4��2+5NaOH $\frac{\underline{\;\;��\;\;}}{\;}$NH3��+3H2O+NaAlO2+2Na2SO4��
��4��������1mol������茶��壬Ħ��������453g/mol��������453g�����õ�������������������茶�����ȹ����л�ֽ⣬�ֱ�������ˮ����������������
���ᾧˮ��ȫʧȥʱ��ʣ�������ԭʼ����������ٷֱ��ǣ�$\frac{18��12}{453}$��100%��52.32%���������ֵ��˵���ᾧˮû����ȫʧȥ��
���¶ȼ������ߣ���������е�����刺�ʼ�ֽ����ɰ��������������������ȫ�ֽ�ʱ��ʣ����Ϊ��������ʣ�������ԭʼ����������ٷֱ��ǣ�$\frac{171}{453}$��100%��37.75%������ֵ����37.75%ʱ�������û����ȫ�ֽ⣬�����������ֵ��˵�������ǡ�÷ֽ���ȫ��С�������ֵ����������ʼ�ֽ⣻��B��ʣ���������������
�ʴ��ǣ�Al2��SO4��3��
��5�����Ϸ���������ͼ��M����Ҫ�ɷֵĻ�ѧʽΪNH3��H2O��SO3 ��������Ũ������������������ظ�ʹ�ã�
�ʴ��ǣ�NH3��H2O��SO3 �� Ũ���ᣮ
���� ���⿼������Al��OH��3��H2SO4����ҵ��NH4��2SO4����FeSO4��Ϊԭ���Ʊ����������մɵķ������ؼ������������������Ϣ��������ѧ��ѧ֪ʶ��ɣ������Ѷ��еȣ�


 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
�� �١��ڡ���3��-OH��������ǿ������˳���ǣ�
�١��ڡ���3��-OH��������ǿ������˳���ǣ� ��
��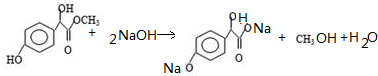 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢ� | B�� | �ڢܢ� | C�� | �ڢۢ� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2���γ��������Ҫ���� | |
| B�� | CO2��������ЧӦ����һ�ִ�����Ⱦ�� | |
| C�� | CO2��g��+C��s��$\stackrel{����}{?}$2CO��g����H��0�����������ڸ÷�Ӧ�Է����� | |
| D�� | ʵ���ҳ��ô���ʯ��ϡ�����ϡ���ᷴӦ��ȡ������̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijѧ����ͨ��ʵ�鷽����֤ Fe2+�����ʣ�
ijѧ����ͨ��ʵ�鷽����֤ Fe2+�����ʣ�| ʵ����� | Ԥ������ | ��Ӧ�����ӷ���ʽ |
| ��ʢ������FeSO4��Һ���Թ��е�������Ũ���ᣬ�� | �Թ��в�������ɫ���壬��Һ��ɫ��� | Fe2++NO${\;}_{3}^{-}$+2H+�TFe3++NO2��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������CO2ͨ��NaOH��Һ�У�CO2+2OH-=CO32 -+H2O | |
| B�� | ��ˮ��ͨ�����CO2��2NH3•H2O+CO2=2NH4++CO32-+H2O | |
| C�� | ����̼�������Һ�м��뱥������������Һ��Ca2++HCO3-+OH-=CaCO3��+H2O | |
| D�� | NaHCO3��Һ�м��������Ca��OH��2��Һ��2HCO3-+Ca2++2OH-=CaCO3��+2H2O+CO32 - |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Ϊ̽��Fe��NO3��2���������ȷֽ����Ͳ�������ʣ�ij��ѧС�鿪չ����̽����ѧϰ��
Ϊ̽��Fe��NO3��2���������ȷֽ����Ͳ�������ʣ�ij��ѧС�鿪չ����̽����ѧϰ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com