
����6�֣������������ʣ� ��NaCl���塡��Һ̬SO2���۴����ᡡ�����ᱵ����ͭ �ƾ���C2H5OH�� ���ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ�������������������������������������������������� ��
��2������������ʵ������������������������������������� ��
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ������������������������������ ��
����4�֣�
ij��ѧʵ��С��̽������ʳ�ð״��д���ĵ�ȷŨ�ȣ�ȡ25.00mLijƷ��ʳ�ð�
������ƿ�У���ʵ������Ũ��Ϊcb mol/L�ı�NaOH��Һ������еζ���
��1����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����l mL��
A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ����������mL��
��2��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ��
��ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ
������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
 H++OH-��KW=10-14�� CH3COOH
H++OH-��KW=10-14�� CH3COOH  H++ CH3COO����Ka=1.8��10-5
H++ CH3COO����Ka=1.8��10-5���ݢߢࣻ�ۣ��ڣ���ÿ��2�֣�
����
��1��25.40 mL��1�֣�
��2��A��B��2�֣�
��3��C��25.35+25.30��/25.00��1�֣�
����1����С��1�֣�
��2��CH3COO�� + H2O CH3COOH+OH-������3�֣�
CH3COOH+OH-������3�֣�
��3��С�ڣ����ڣ�2�֣�
��4��c��Na+��>c��CH3COO-��>c��OH-��>c��H+����2�֣�
��5�����ڣ�1�֣�
��6��AD��2�֣�
��7��Cd2+��Zn2+����1�֣�
��OH-��=2.2��10-5mol��L-1����
��M2+�ݣ�OH-��2=5��10-12��mol��L-1��3
5��10-12��KSP��Mg��OH��2��=1.8��10-11
5��10-12����KSP��Zn��OH��2��=1.2��10-17
5��10-12����KSP��Cd��OH��2��=2.5��10-14��2�֣�
��8��1��10-12
�������⿼����ǵ������Һ���������ĵ�����ˮ�⣬�ⲿ�����ݵ�����ѣ�����������������ʡ�ˮ�⼰�����ܽⶼ�ǿ���ģ��������漰����ѧƽ�ⷽ������ݣ����鷶Χ�Ϲ㡣
�������ϼ����ﲻ��������������Ϊ����3С��Ĵ�����Ӧ����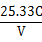
����
��1�� ������Һ�д��ڵ���ƽ�⣺CH3COOH  H++ CH3COO��������������ƹ���ʱ��CH3COO��Ũ��������ƽ�������ƶ������½������C��H+����С��C��CH3COOH����������C��H+����C��CH3COOH���ı�ֵ��С��
H++ CH3COO��������������ƹ���ʱ��CH3COO��Ũ��������ƽ�������ƶ������½������C��H+����С��C��CH3COOH����������C��H+����C��CH3COOH���ı�ֵ��С��
��2�� CH3COO�� + H2O CH3COOH+OH-��ˮ�������ȵģ����¶�������ƽ�������ƶ�������C(OH��)����
CH3COOH+OH-��ˮ�������ȵģ����¶�������ƽ�������ƶ�������C(OH��)����
��3�� ��������Һ����ˮ��ƽ�⣺CH3COO�� + H2O CH3COOH+OH-��1mol��L-1��������Һ�൱����0��5mol��L-1�����ϼ�������ƣ�ˮ��ƽ�������ƶ���C(OH��) ���ߣ�����m<n,�����ֻ�ѧƽ��ֻ�����ƴ����Ƽ����Ӱ�죬�����ܵ���������ˮ��ȵĶ��壬����a>b��
CH3COOH+OH-��1mol��L-1��������Һ�൱����0��5mol��L-1�����ϼ�������ƣ�ˮ��ƽ�������ƶ���C(OH��) ���ߣ�����m<n,�����ֻ�ѧƽ��ֻ�����ƴ����Ƽ����Ӱ�죬�����ܵ���������ˮ��ȵĶ��壬����a>b��
��4�� �������Ũ�ȵĴ��������������Һ������ɴ����ƣ��ʼ��ԣ�����C(Na+)>C(CH3COO��)> C(OH��)> C(H+)����Һ�д��ڵ���غ㣩
��5�� ���ݵ���غ㣬C(OH��)<C(H+),��Ȼ��C(Na+)<C(CH3COO��)
��6�� ���˾���������������ȷ��Ӧ����B��C�������������pH��3��HA��Һ��pH��11��NaOH����ҺpH<7,������Ϊ������������ʣ�������ӻ��ڻ�Ϲ����е����H+,����B��C˵������ȷ��
��7�� ��8����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com