
��15�֣�����ѧ��ѡ��5���л���ѧ������
��֪�л���A��I֮���ת����ϵ����ͼ��ʾ��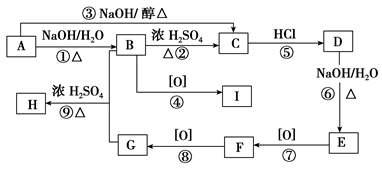
��֪��
����A��D��B��E��I��F��Ϊͬ���칹��
���ڽ�����Cu(OH)2����Һ�ֱ���뵽�л���I��F�У����ȣ�I����������F��Ӧ������ש��ɫ����
����C��ʵ��ʽ����Ȳ��ͬ������Է�������Ϊ104
�� ��E��һ��ͬ���칹����FeCl3�ܷ�����ɫ��Ӧ
����������Ϣ���ش��������⣺
��1��H�� F�к��еĺ������������Ʒֱ�Ϊ �� ��
��2����Ӧ�١�����������ȥ��Ӧ����____________________________��
��3��I�Ľṹ��ʽΪ____________________________��
��4��д��H������������ˮ��Ļ�ѧ����ʽ ��
��5��д��F��������Һ��Ӧ�Ļ�ѧ����ʽ ��
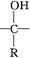
��6����������������G��ͬ���칹����________�֣����б�����ֻ��2��һԪȡ����Ľṹ��ʽΪ ��
�����ڷ����廯����
������NaOH��Һ��Ӧ
���ܷ���������Ӧ
������ע�⣬ÿ��2�֣���15�֣�
��1��������1�֣� ȩ����1�֣�
��2���ڢۣ���һ����1�֣��д���0�֣�
��3�� 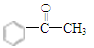 �� C6H5COCH3
�� C6H5COCH3
��4��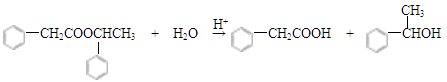
��5��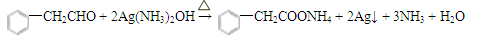
��6�� 14 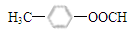 (3��)
(3��)
�������������A�����������ƵĴ���Һ�з�����ȥ��Ӧ˵��A��±����������BΪ����B��C������ȥ��Ӧ��C��ʵ��ʽ����Ȳ��ͬ������Է�������Ϊ104����C�ķ���ʽΪC8H8��CΪ���������������ӳɷ�Ӧ����DΪ±������EΪ����E��һ��ͬ���칹����FeCl3�ܷ�����ɫ��Ӧ��˵��E�к��б���������C�DZ���ϩ��FΪ����ȩ��GΪ�����ᣬ��H������I��B�������������I��ȩ��ͪ��I��F��Ϊͬ���칹�壬����I��ͪ���������Ϸ�����
��1��H�� F�к��еĺ������������Ʒֱ�Ϊ������ȩ����
��2����Ӧ�١�����������ȥ��Ӧ���Ǣڢۣ�
��3��I��ͪ���뱽��ȩ��Ϊͬ���칹�壬����I�Ľṹ��ʽΪ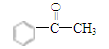
��4��H�DZ�������B���ɵ�������I�Ľṹ��ʽ�ж�B���ǻ��������ͬһCԭ���ϣ�����Hˮ��Ļ�ѧ����ʽΪ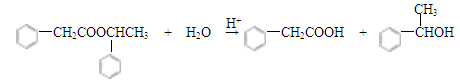
��5��F�DZ���ȩ����������Һ��Ӧ�Ļ�ѧ����ʽΪ
��6��G�DZ����ᣬ����NaOH��Һ��Ӧ��˵�������д����������Ȼ�����ǻ�����������Һ��Ӧ��˵�������д���ȩ�����ۺ��������������������G��ͬ���칹���Ǽ���ij�������ȩ�������ǻ��ͼ�������ǰ�ߣ�����4�ֲ�ͬ�ṹ����Ϊ���ߣ�����ȡ������ͬ���칹����жϿ����ȹ̶�2��ȡ������Ȼ���жϱ�������ԭ�ӵ����࣬���Թ���10�֣���˷���������G��ͬ���칹�干��14�֣����б�����ֻ��2��һԪȡ����Ľṹ��ʽΪ��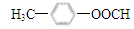
���㣺�����л�����ƶϣ������š���Ӧ���͡��ṹ��ʽ����ѧ����ʽ���жϣ�ͬ���칹����жϼ���д



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ�У��л��ﱻ��ԭ����
����ȩ���Ҵ� ����ȩ������ ����Ȳ����ȩ ���Ҵ�����ȥ
����Ȳ������ ���Ҵ�����ȩ
| A���٢� | B���ܢ� | C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���A��B��C��D��E��F��G��H�ת����ϵ����ͼ��ʾ��5.2 g F����100 mL 1 mol/L NaOH��Һǡ����ȫ�кͣ�0.1 mol F����������NaHCO3��Ӧ���ڱ�״���·ų�4.48 L CO2��D�ķ���ʽΪC3H3O2Na��E�ķ����к����Ȼ���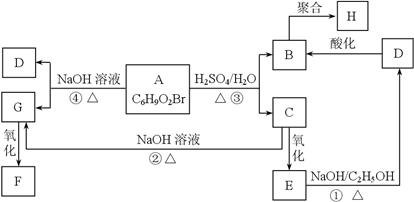
��1��д������C�еĹ����ŵ����ƣ� ��
��2��д������F��H�Ľṹ��ʽ��
F ��H ��
��3��д����Ӧ�١��ܵĻ�ѧ��Ӧ���ͣ��� ���� ��
��4��д���仯�١��۵Ļ�ѧ����ʽ��
��
��
��5��д����Է���������B��14������B������ͬ�����ŵ��������ʵĽṹʽ�������������칹����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
[��ѧ���л���ѧ����]��13�֣�
ij�о�С���Լױ�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�ҽҩ�м���F��Y��
��֪��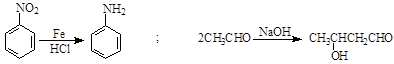 ��
��
��ش��������⣺
��1��д��Y�к��������ŵ����� ��
��2�������й�F��˵����ȷ���� ��
| A������ʽ��C7H7NO2Br | B�����������ᷴӦ������NaOH��Һ��Ӧ |
| C���ܷ���������Ӧ | D��1 mol F����������2 mol NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ϩ��ʯ�ͻ�������Ҫ�Ļ���ԭ�ϣ���������¿�ͼ�ش�
41.��ʯ��Ϊԭ�ϵ�ϵ�л������������У��õ�������ϩ����Ҫ������_______��ѡ����ţ���
a. ˮ�� b. ���� c. �ѽ� d. �ѻ�
42.�л���A�׳�______________�����еĹ�����������_________________.
43.B�ķ���ʽΪC2H4O2���봿�Ӧ�����ɶ�����̼���壬д����ӦA��B��C�Ļ�ѧ
����ʽ___________________________________________________________ ���л����ýṹ��ʽ��ʾ�����÷�Ӧ����Ϊ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
[��ѧ����ѡ��5���л��ﻯѧ����]��15�֣�
�߷��Ӳ���E�ͺ�����Ϣʹ�߷���ҩ��ĺϳ�������ͼ��ʾ��
��֪��I��������Ϣʹ�߷���ҩ��ĽṹΪ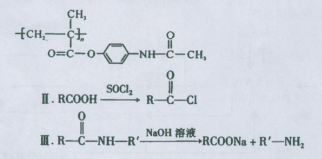
�Իش��������⣺
��1����Ӧ�ٵķ�Ӧ����Ϊ ��G�ķ���ʽΪ ��
��2����1mol CH�� CH3��2��ת��Ϊ1mol A��1mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
CH�� CH3��2��ת��Ϊ1mol A��1mol B����A��FeCl3��Һ��������ɫ��д��A��ϡ��Һ�����Ũ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
��3����Ӧ��Ϊ�ӳɷ�Ӧ����B�Ľṹ��ʽΪ ������Ϣʹ�Ľṹ��ʽΪ ��
��4��D�����ܶ�����ͬ״̬�¼����ܶȵ�6��25����D�и�Ԫ�ص����������ֱ�Ϊ̼60%����8%����32%��D����������������Ϊ ��
��5��д��������Ϣʹ�߷���ҩ��������������Һ������Ӧ�Ļ�ѧ����ʽΪ ��
��6��D�ж���ͬ���칹�壬������D������ͬ���������ܷ���������Ӧ��ͬ���칹����
�֣�����˳���칹����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��18�֣�����2.8g�л���A����ȫȼ������0.15molCO2��1.8gH2O��A������ͼ����ͼ��ʾ����֪��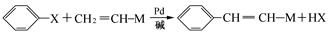 ��XΪ±ԭ�ӣ�MΪ������������ȡ������)�����л���A�ϳ�G���㶹�أ��IJ������£�
��XΪ±ԭ�ӣ�MΪ������������ȡ������)�����л���A�ϳ�G���㶹�أ��IJ������£�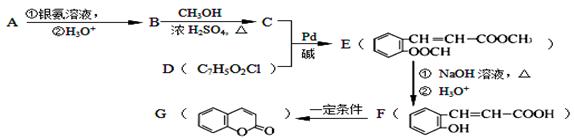
�ش��������⣺
��1��A�ķ���ʽΪ ��
��2��д��C�к�������������:�� ����F��G �ķ�Ӧ������ ��
��3��д��A��������Һ��Ӧ�Ļ�ѧ����ʽ�� ����
��4��D�Ľṹ��ʽΪ�� ����
��5�������㶹�أ� 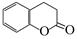 ���������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨
���������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨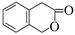 ����Ҫ�õ����Լ��У�NaOH��Һ���� ����
����Ҫ�õ����Լ��У�NaOH��Һ���� ����
��6��F�ж���ͬ���칹�壬д��ͬʱ������������������ͬ���칹��Ľṹ��ʽ�� ����
��. �����г������⣬��������״�ṹ�� ��.���������������ڶ�λ��ȡ������
��. �ܷ���ˮ�ⷴӦ��������Na��Ӧ�� ��.��������Cu(OH)2�����ʵ�����1:2����������Ӧ
��7����֪��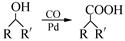 (R��R��Ϊ����)����д���Ա��ͱ�ϩ��
(R��R��Ϊ����)����д���Ա��ͱ�ϩ��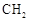 ��CH��CH3��Ϊԭ�ϣ��ϳ�
��CH��CH3��Ϊԭ�ϣ��ϳ�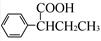 ��·������ͼ���£�
��·������ͼ���£�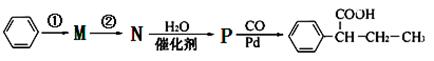
����ٵķ�Ӧ�������Լ�____________������ڵķ�Ӧ����____________��P�Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������A�Ƿ���ʽΪC7H8O2����Ԫ��״������,���������ֲ�ͬ��ѧ��������,��ԭ�Ӹ�����Ϊ3��1��2��2,���ܷ�������ת��: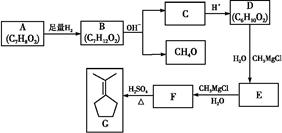
��֪:�ٺ��ʻ��Ļ�����������ʽ�Լ��������·�Ӧ: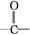
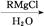
�������ǻ�����ͬһ̼ԭ���ϼ����ȶ�,����ˮ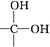
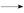
 +H2O
+H2O
����������Ϣ,�Իش���������:
(1)д��������A�й����ŵ����� ��
(2)д��������B��F�Ľṹ��ʽB ,F ��
(3)A��B�ķ�Ӧ������ ,F��G�ķ�Ӧ������ ��
(4)��д��D��CH4O��Ӧ�Ļ�ѧ����ʽ ��
��д��F��G�ķ�Ӧ�Ļ�ѧ����ʽ ��
(5)д��������A�ķ�������������ͬ���칹�� ��
�����ڷ����廯���� ������̼������Һ��Ӧ �����ڴ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����(CH3)3COH�ṹ���ƵĴ����ܱ������ɶ�Ӧ��ȩ�����ᡣ������������AΪ��ʼ��Ӧ��ϳ�PMAA��·�ߣ�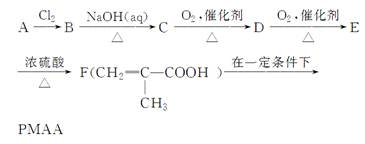
����д���пհף�
(1)A�Ľṹ��ʽΪ�� ��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
E��F�� .
F��PMAA�� ��
(3)E����Ũ���Ტ���ȵ������£���������F�⣬����������һ�ַ�������һ����Ԫ�����л���G��G�Ľṹ��ʽΪ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com