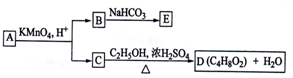
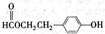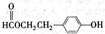ΆΦ÷– AΓΔBΓΔCΓΔDΓΔEΨυΈΣ”–ΜζΜ·ΚœΈοΘ°“―÷ΣΘΚCΡήΗζNaHCO
3ΖΔ…ζΖ¥”ΠΘ§CΚΆDΒΡœύΕ‘Ζ÷Ή”÷ ΝΩœύΒ»Θ§«“EΈΣΈό÷ßΝ¥ΒΡΜ·ΚœΈοΘ°
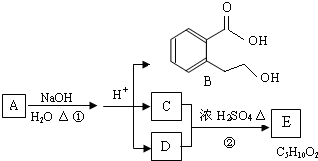
ΗυΨίΆΦΜΊ¥πΈ ΧβΘΚ
Θ®1Θ©CΖ÷Ή”÷–ΒΡΙΌΡήΆ≈Οϊ≥Τ «ΘΚ
τ»Μυ
τ»Μυ
ΘΜ
œ¬Ν–Ζ¥”Π÷–Θ§Μ·ΚœΈοB≤ΜΡήΖΔ…ζΒΡΖ¥”Π «
e
e
Θ®ΧνΉ÷ΡΗ–ρΚ≈Θ©ΘΚ
aΓΔΦ”≥…Ζ¥”Π bΓΔ»Γ¥ζΖ¥”Π cΓΔœϊ»ΞΖ¥”Π dΓΔθΞΜ·Ζ¥”Π eΓΔΥ°ΫβΖ¥”Π fΓΔ÷ΟΜΜΖ¥”Π
Θ®2Θ©Ζ¥”ΠΔΎΒΡΜ·―ßΖΫ≥Χ Ϋ «
CH
3COOH+CH
3CH
2CH
2OH
CH
3COOCH
2CH
2CH
3+H
2O
CH
3COOH+CH
3CH
2CH
2OH
CH
3COOCH
2CH
2CH
3+H
2O
Θ°
Θ®3Θ©AΒΡΫαΙΙΦρ Ϋ «
Θ°
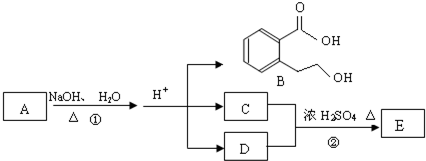
Θ®4Θ©Ά§ ±ΖϊΚœœ¬Ν–»ΐΗωΧθΦΰΒΡBΒΡΆ§Ζ÷“λΙΙΧεΒΡ ΐΡΩ”–
4
4
ΗωΘ°
ΔώΘ°Κ§”–ΦδΕΰ»Γ¥ζ±ΫΜΖΫαΙΙΘΜΔρΘ° τ”ΎΖ«ΖΦœψΥαθΞΘΜΔσΘ°”κ FeCl
3 »ή“ΚΖΔ…ζœ‘…ΪΖ¥”ΠΘ°
–¥≥ωΤδ÷–»Έ“β“ΜΗωΆ§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ Ϋ
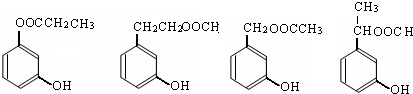
–¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω…

–¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω…
Θ®5Θ©≥ΘΈ¬œ¬Θ§ΫΪC»ή“ΚΚΆNaOH»ή“ΚΒ»ΧεΜΐΜλΚœΘ§ΝΫ÷÷»ή“ΚΒΡ≈®Ε»ΚΆΜλΚœΚσΥυΒΟ»ή“ΚpH»γœ¬±μΘΚ
| Β―ι±ύΚ≈ |
CΈο÷ ΒΡΝΩ≈®Ε»Θ®mol?L-1Θ© |
NaOHΈο÷ ΒΡΝΩ≈®Ε»Θ®mol?L-1Θ© |
ΜλΚœ»ή“ΚΒΡpH |
| m |
0.1 |
0.1 |
pH=9 |
| n |
0.2 |
0.1 |
pHΘΦ7 |
¥”mΉι«ιΩωΖ÷ΈωΘ§ΥυΒΟΜλΚœ»ή“Κ÷–”…Υ°Βγάκ≥ωΒΡcΘ®OH
-Θ©=
10-5
10-5
mol?L
-1Θ°
nΉιΜλΚœ»ή“Κ÷–άκΉ”≈®Ε»”…¥σΒΫ–ΓΒΡΥ≥–ρ «
cΘ®CH3COO-Θ©ΘΨcΘ®Na+Θ©ΘΨcΘ®H+Θ©ΘΨcΘ®OH-Θ©
cΘ®CH3COO-Θ©ΘΨcΘ®Na+Θ©ΘΨcΘ®H+Θ©ΘΨcΘ®OH-Θ©
Θ°



 –Γ―ßΆ§≤Ϋ»ΐΝΖΚΥ–ΡΟήΨμœΒΝ–¥πΑΗ
–Γ―ßΆ§≤Ϋ»ΐΝΖΚΥ–ΡΟήΨμœΒΝ–¥πΑΗ







 –¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω…
–¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω… –¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω…
–¥≥ωΥΡ’Ώ÷°“ΜΦ¥Ω…