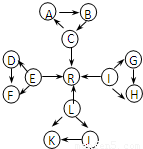
Ԫ�ؼ��仯��������ѧ��ѧ������֪ʶ������������Ԫ�ػ�����֮����ת��ʱ��������Щ���ʼ������ͼ��ʾ������ת����ϵ�����ַ�Ӧ�������������ȥ����
��֪��
��ͼ��ÿ��С�����ε��������������ٺ���һ����ͬԪ�أ�A��B��D��F��K��J����2����ͬ��Ԫ����ɣ�
��C��E��L��IΪ�������嵥�ʣ�C��E��L������ˮ��һ�������·�Ӧ�������嵥��R��D��F�������ķǽ���Ԫ�����γɵĵ��ʳ�����Ϊ����ɫ���壮��������Ϊ���������
��KΪ����ɫ���壮
��C��L��I��Ԫ��ԭ�ӵ�ԭ������֮��Ϊ30��
����������Ϣ���ش��������⣺
��1���R������
����
����
��K�ĵ���ʽ
��
��2����Щ����B����C�Ļ�ѧ��Ӧ����ʽ
��
��3��I������������Һ��Ӧ�����ӷ���ʽ
2Al+2OH-+2H2O=2AlO2-+3H2��
2Al+2OH-+2H2O=2AlO2-+3H2��
��I������������Һ��Ӧ������R�����Եõ�H���������ӷ���ʽ��ʾHˮ��Һ�ʼ��Ե�ԭ��
AlO2-+2H2O?Al��OH��3+OH-
AlO2-+2H2O?Al��OH��3+OH-
��
��4���ö��Ե缫���һ��Ũ��������L���Ȼ���ˮ��Һ����������ʱ��ͨ������Ϊ0.2mol������ָ������£���Һ�����Ϊ2L������Һ��pHΪ
13
13
��
��5����D��Һ�к�������F����γ�ȥ��
��DΪFeCl2����ͨ��������Cl2���ɣ���DΪFeCl3�������������Fe���ٹ���
��DΪFeCl2����ͨ��������Cl2���ɣ���DΪFeCl3�������������Fe���ٹ���
��

 Ԫ�ؼ��仯��������ѧ��ѧ������֪ʶ������������Ԫ�ػ�����֮����ת��ʱ��������Щ���ʼ������ͼ��ʾ������ת����ϵ�����ַ�Ӧ�������������ȥ����
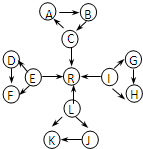
Ԫ�ؼ��仯��������ѧ��ѧ������֪ʶ������������Ԫ�ػ�����֮����ת��ʱ��������Щ���ʼ������ͼ��ʾ������ת����ϵ�����ַ�Ӧ�������������ȥ����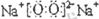
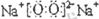
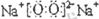 ���ʴ�Ϊ��������
���ʴ�Ϊ��������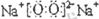 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
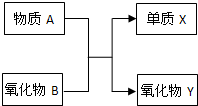 Ԫ�ؼ��仯�����֪ʶ�ǡ���ѧI�����ص����ݣ� A��B��X��Y��Ϊ��ѧ�εij������ʣ�����֮���ת����ϵ��ͼ��ʾ��
Ԫ�ؼ��仯�����֪ʶ�ǡ���ѧI�����ص����ݣ� A��B��X��Y��Ϊ��ѧ�εij������ʣ�����֮���ת����ϵ��ͼ��ʾ��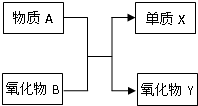 Ԫ�ؼ��仯�����֪ʶ�ǡ���ѧI�����ص����ݣ� A��B��X��Y��Ϊ��ѧ�εij������ʣ�����֮���ת����ϵ��ͼ��ʾ��
Ԫ�ؼ��仯�����֪ʶ�ǡ���ѧI�����ص����ݣ� A��B��X��Y��Ϊ��ѧ�εij������ʣ�����֮���ת����ϵ��ͼ��ʾ��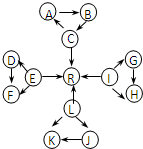 Ԫ�ؼ��仯��������ѧ��ѧ������֪ʶ������������Ԫ�ػ�����֮����ת��ʱ��������Щ���ʼ������ͼ��ʾ������ת����ϵ�����ַ�Ӧ�������������ȥ����
Ԫ�ؼ��仯��������ѧ��ѧ������֪ʶ������������Ԫ�ػ�����֮����ת��ʱ��������Щ���ʼ������ͼ��ʾ������ת����ϵ�����ַ�Ӧ�������������ȥ����