
ij������ˮ��Һ�����ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2����NH4+��Cl����CO32����SO42�����ֱַ�ȡ100 mL�����ȷ���Һ��������ʵ�飺
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02 g���塣
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65 g���塣
(1)һ�������ڵ�������________(�����ӷ��ţ���ͬ)��
(2)�ɢٿ�֪��������Ϊ________��Ũ��________���ɢڿ�֪��������Ϊ________��Ũ��________��
�ɢۿ�֪��������Ϊ________��Ũ��________��
(3)K���Ƿ���ڣ�________(��ǡ���)��������___________________________
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4molA�����2molB������2L���ܱ������л�ϣ���һ�������·������·�Ӧ��
2A(g)+B(g) 2C(g)������2�����C��Ũ��Ϊ0.6mol/L��
2C(g)������2�����C��Ũ��Ϊ0.6mol/L��
��1��2s����B��ʾ�ķ�Ӧ���� ��
��2��2sʱA�����ʵ���Ũ��Ϊ ��
��3��2sʱB�����ʵ���Ϊ ��
��4������C��Ũ�� ����ܡ����ܡ����ﵽ2mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)2 mol O3��3 mol O2������֮�� ��������֮�� ��ͬ��ͬѹ�µ��ܶ�֮�� ������ԭ����֮�� �����֮�� ��
(2)O3��Cl2�������Ƶ����ʣ�������������ˮ����������֪����������ʱ������ԭΪ��ͼ�̬������ͬ״����10 L O3�� L Cl2�����������൱��
(3)���廯����A����ʽ�ɱ�ʾΪOxFy����֪ͬ��ͬѹ��10 mL A���ȷֽ�����15 mL O2��10 mL F2����A�Ļ�ѧʽΪ ���ƶϵ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
| | ���ʵ����ʵ��� Ũ��/mol��L��1 | ��Һ���ܶ�/g��cm��3 |
| ���� | c1 | ��1 |
| ��ˮ | c2 | ��2 |
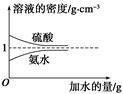
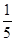 c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________
c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________ 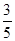 c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��
c2 mol��L��1(���Ϻ���Һ������仯���Բ���)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ��������90mL 2mol��L-̼������Һ��
��1�����Ƹ���Һʱ���������ʵ�����飬���������������õ�����_____����ѡ���
A. ������ƽ B. �ձ� C. ��ͷ�ι� D. 100ml����ƿ E. 90ml����ƿ F.������
��2��ʵ����������У�A.��ȡ̼���ƾ��壻B.��������ˮ���ձ�������ϴ��2��3�Σ�ÿ��ϴ��ҺҲת�Ƶ�����ƿ��C.���ձ��е���Һת�Ƶ�ѡ��������ƿ�У�D.��̼���ƾ��������ձ�����������ˮ�ܽ⣬���ò�����������ȣ�E.����õ�̼������Һװ���Լ�ƿ���ò����ñ�ǩ��F.������ƿ��ˮ���̶���1-2cm�����ý�ͷ�ιܵμ�ˮ��Һ����̶������У�G.����ƿ�����������µߵ�ҡ�ȡ�
�������������ȷ����˳���� B ����д��ĸ��
�ڱ�ʵ���ȡ��̼���ƾ���������� g
��������ʱ���ӿ̶��ߣ���������ҺŨ�� ���ƫ����ƫС������Ӱ�족��
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ����������� ����ѡ���
A. ��������Һ�壬ʹ��Һ����̶�������
B. С�ļ�������ƿ��Һ����������ʹ��Һ����̶�������
C. ��ȷ�������һ������Ũ����
D. ��������̼������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������£���ijһ�ݻ��̶�����������ڳ��붡�飨������������ʹ�����ڻ��������ѹǿ�ﵽp1�����ȼ�գ�������Ӧ��ȫ����ȴ�����º��������������ѹǿΪp2��
��1��������ȼ�յ�������ֻ��H2O��Һ����CO2����p2/p1�� ��
��2����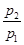 ��0.64����Ӧǰ������ж�������ʵ��������� ��
��0.64����Ӧǰ������ж�������ʵ��������� ��
�����谢���ӵ�����ΪNA���ڳ��³�ѹ�������Ħ�����ΪVm L��mol��1��O2��N2�Ļ������a g����b�����ӣ���c g�û�������ڳ��³�ѹ����ռ�����Ӧ�� L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�������ﺬNH4I��NaHCO3��AlCl3��MgBr2��FeCl2�еļ���,Ϊȷ���ù�������ijɷּ�����ɳɷֵ����ʵ���֮��,�ֽ�������ʵ�顣
ʵ���:
(1)��ɫ����Ϊ ��
(2)�ù�������ijɷ�Ϊ ��
ʵ���:ȡһ�����ĸù�����������ˮ���1 L��Һ,����û����Һ��ͨ��һ������Cl2,�����Һ�м���������(�ֱ���A-��B-��C-��ʾ)�����ʵ�����ͨ��Cl2����Ĺ�ϵ�����ʾ��
| Cl2����� (��״����)/L | 2.8 | 5.6 | 11.2 |
| n(A-)/mol | 1.25 | 1.5 | 2 |
| n(B-)/mol | 1.5 | 1.4 | 0.9 |
| n(C-)/mol | a | 0 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��ش��������⡣
��1��SO2���γ������������ס� 64 g SO2�����ʵ���Ϊ ���ڱ�״���µ����ԼΪ ������ԭ����Ϊ ��
��2��д����ȥ���������������ʣ�������Ϊ���ʣ����õ��Լ�����
��Na2CO3����(NaHCO3) ��FeCl3��Һ(FeCl2)
��Mg�ۣ�Al�� ��CO2(SO2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͬ��ͬѹ�£���0.3molO2��0.2molO3�����ǵ�����֮��Ϊ ������������ԭ����֮��Ϊ �����ǵ����֮��Ϊ �����ǵ��ܶ�֮��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com