
Ŀǰ����ѧ�������о�����һ�ָ��ܵ�ء��������أ��������ڵ��ơ���Ϊ��������Na+����Ħ£�Al2O3�մ����������ʣ���Ӧʽ���£�2Na��xS Na2Sx������˵������ȷ����
Na2Sx������˵������ȷ����
A���ŵ�ʱNa��������S��������ԭ��Ӧ
B�����ʱ���Ƽ������Դ����������
C�����ʱ������ӦΪ��xS2--2e-��xS
D�����ŵ�ʱת��0��2 mol ���ӣ�����ȥ����Ϊ4��6g
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| 16.8+11.2x |
| x |
| 16.8+11.2x |
| x |
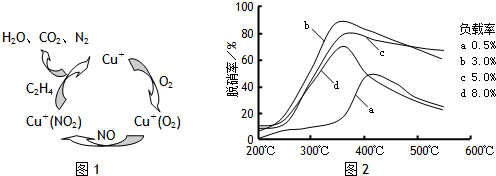
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��X��Y��Z�Ƕ�����Ԫ�ص����ֳ��������X��ˮ��Ӧ������һ�־��л�ԭ�ԵIJ��ȶ��Ķ�Ԫ�ᣬ����Ļ�ѧʽ��
��X��Y��Z�Ƕ�����Ԫ�ص����ֳ��������X��ˮ��Ӧ������һ�־��л�ԭ�ԵIJ��ȶ��Ķ�Ԫ�ᣬ����Ļ�ѧʽ��
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| 16.8+11.2x |
| x |
| 16.8+11.2x |
| x |
| ||
| ||
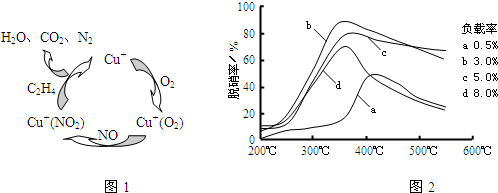
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
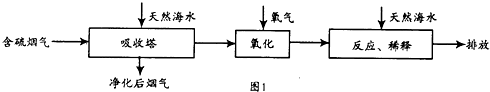
| ʵ����� | �¶ȣ�K�� | ��������SO2Ũ�ȣ�10-6mol��l-1�� | �����ʣ�%�� |
| �� | 298 | 400 | 99.5 |
| �� | T | 500 | 97.1 |
| �� | 313 | 400 | 94.3 |
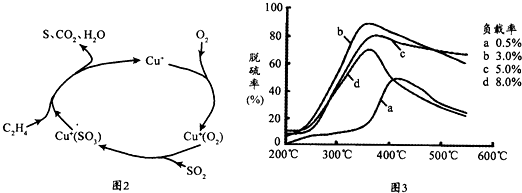
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com