
��10�֣���ϩ��Һ��������ȡ1��2�D���������װ�����£�D���Թ�����װ����ΪҺ�壩
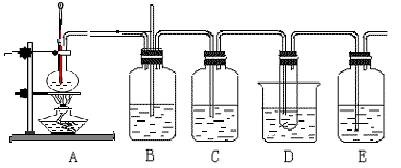
1��2�D�����������Ҫ����������
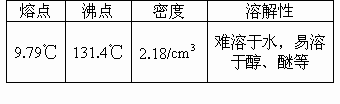
�۵� | �е� | �ܶ� | �ܽ��� |
9.79�� | 131.4�� | 2.18g?cm-3 | ������ˮ�������ڴ����ѵ� |
��1��A���Ҵ��Ʊ���ϩ�ķ�Ӧװ��ͼ��B��D����ʢ����ˮ������Dװ����ˮ��������
______________________________________________
��2��C��E��ʢ����NaOH��Һ�����ǵ����÷ֱ��ǣ�
C_________________________________��E_____________________________________
��3��д�����Ҵ���Ũ���ᡢҺ��Ϊ��Ҫԭ������1��2�D�����������Ҫ��ѧ��Ӧ����ʽ________________________________ ________________
___________________________________ _____________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �۵� | �е� | �ܶ� | �ܽ��� |
| 9.79�� | 131.4�� | 2.18g?cm-3 | ������ˮ�������ڴ����ѵ� |
| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
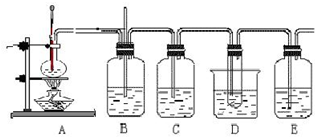 ��ϩ��Һ��������ȡ1��2-���������װ�����£���D���Թ�����װ����ΪҺ�壩
��ϩ��Һ��������ȡ1��2-���������װ�����£���D���Թ�����װ����ΪҺ�壩| �۵�/�� | �е�/�� | �ܶ�/g?cm-3 | �ܽ��� |
| 9.79 | 131.4 | 2.18 | ������ˮ�������ڴ����ѵ� |
| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��֪1��2�������������Ҫ�����������£�
��ͼΪʵ��������ϩ��Һ��������ȡ1��2����������IJ���װ��ͼ��
(1)A��C�ж�ʢ��ˮ��������װ�õ����÷ֱ���_______________________
(2)B��Dװ�ö�ʢ��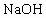 ��Һ�����ǵ����÷ֱ���________________��
��Һ�����ǵ����÷ֱ���________________��
(3)д����ϩ��Һ�巴Ӧ����1��2�������������Ҫ��ѧ��Ӧ����ʽ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ����ʦ����ѧ����2006~2007ѧ��ȵڶ�����ĩ���ԡ��߶���ѧ���� ���ͣ�058
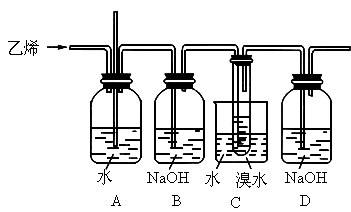
ʵ�飺��ͼΪʵ��������ϩ��Һ��������ȡ1��2�����������װ��ͼ��
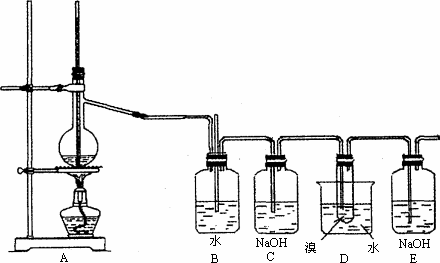
1��2�������������Ҫ����������

(1)B��D�ж�ʢ��ˮ��������װ�õ����÷ֱ���______________��_____________��
(2)C��Eװ�ö�ʢ��NaOH��Һ�����ǵ�������________________��
(3)д�����Ҵ���Ũ���ᡢҺ��Ϊ��Ҫԭ������1��2�������������Ҫ��ѧ��Ӧ����ʽ________________��________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com