
��10�֣�ʵ���Ҷ�������ij��Ʒ���������Ƶ�һ�ַ����ǣ�
����1.520g��Ʒ�м���̼�������Һ��0.13�� I2���ȷ���Һ���ڷ�Һ©������15min�����ӷ���ʽΪ��SO32����I2��2HCO3����SO42����2I����2CO2����H2O
��ȡ�������õ�ˮ��Һ������һ�������ᡢ�����ı�����ˮ��Һ����������е����ӱ������ɵ�������ӣ��õ�250mL��Һ��
���ڢ�������Һ��ȡ25mL���μӼ��ᣬ��ȥ���й�����Br2��
�ܽ���������Һ�м������Ĵ����ƣ��ټ��������ĵ⻯����Һ������Һ�����ӷ���ʽΪ��6H����IO3����5I����3I2��3H2O
���ñ��������������Һ�ζ�����������Һ��������0.1120mol/L Na2S2O3 15.10mL�����ӷ���ʽΪ��I2��252O32����2I����S4O62��
�ش��������⣺
��1��д���ڡ���������������������Ӧ�����ӷ���ʽ��
��2������ΪʲôҪ��0.13�� I2���ȷ���Һ������ֱ����I2��ˮ��Һ��
��3��������Ʒ���������Ƶ������ٷֺ�����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
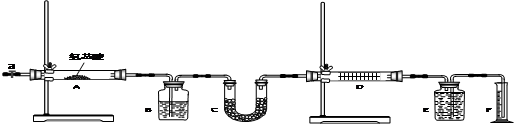
| Bװ�õ�������g�� | Cװ�õ�������g�� | Dװ�õ�������g�� | |
| ʵ��ǰ | 15.4 | 262.1 | 223.8 |
| ����� | 6.1 | 264.8 | 230.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007��1�½���ʡ�����ѧ������ѧ��ĩ�ۺ���ϰ�����˽� ���ͣ�038
ʵ���Ҷ�������ij��Ʒ���������Ƶ�һ�ַ����ǣ�
����1.520 g��Ʒ�м���̼�������Һ��0.13����I2���ȷ���Һ���ڷ�Һ©������15 min�����ӷ���ʽΪ��
SO32����I2��2HCO3����SO42����2I����2CO2����H2O��
��ȡ�������õ�ˮ��Һ������һ�������ᡢ�����ı�����ˮ��Һ����������е����ӱ������ɵ�������ӣ��õ�250 mL��Һ��
���ڢ�������Һ��ȡ25 mL���μӼ��ᣬ��ȥ���й�����Br2��
����ʽΪ��Br2��HCOOH��2HBr��CO2��
�ܽ���������Һ�м������Ĵ����ƣ��ټ��������ĵ⻯����Һ������Һ��
���ñ��������������Һ�ζ�����������Һ��������0.1120 mol/L��Na2S2O3��15.10 mL�����ӷ���ʽΪ��I2��2S2O32����2I����S4O62�����ش��������⣺
(1)д���ڡ���������������������Ӧ�����ӷ���ʽ��
(2)����ΪʲôҪ��0.13����I2���ȷ���Һ������ֱ����I2��ˮ��Һ��
(3)������Ʒ���������Ƶ������ٷֺ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����1.520 g��Ʒ�м���̼�������Һ��0.13% I2���ȷ���Һ���ڷ�Һ©������15 min�����ӷ���ʽΪ��![]() +I2+2
+I2+2![]() ====
====![]() +2I-+2CO2��+H2O
+2I-+2CO2��+H2O
��ȡ�������õ�ˮ��Һ������һ�������ᡢ�����ı�����ˮ��Һ����������е����ӱ������ɵ�������ӣ��õ�250 mL��Һ��
���ڢ�������Һ��ȡ25 mL���μӼ��ᣬ��ȥ���й�����Br2��
����ʽΪ��Br2+HCOOH====2HBr+CO2��
�ܽ���������Һ�м������Ĵ����ƣ��ټ��������ĵ⻯����Һ������Һ��
���ñ��������������Һ�ζ�����������Һ��������0.1120 mol��L-1 Na2S2O3 15.10 mL�����ӷ���ʽΪ��I2+2![]() ====2I-+
====2I-+![]()
�ش��������⣺
(1)д���ڢ�������������������Ӧ�����ӷ���ʽ��
(2)����ΪʲôҪ��0.13% I2���ȷ���Һ������ֱ����I2��ˮ��Һ��
(3)������Ʒ���������Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����1.520 g��Ʒ�м���̼�������Һ��0.13%I2���ȷ���Һ���ڷ�Һ©������15 min�����ӷ���ʽΪ��
![]() +I2+2
+I2+2![]() ====
====![]() +2I-+2CO2��+H2O
+2I-+2CO2��+H2O
��ȡ�������õ�ˮ��Һ������һ�������ᡢ�����ı�����ˮ��Һ����������е����ӱ������ɵ�������ӣ��õ�250 mL��Һ��
���ڢ�������Һ��ȡ25 mL���μӼ��ᣬ��ȥ���й�����Br2������ʽΪ:Br2+HCOOH====2HBr+CO2��
�ܽ���������Һ�м������Ĵ����ƣ��ټ��������ĵ⻯����Һ������Һ��
���ñ��������������Һ�ζ�����������Һ��������0.112 0 mol��L-1 Na2S2O315.10 mL��
���ӷ���ʽΪ��I2+2![]() ====2I-+
====2I-+![]()
����������⣺
��1��д���ڢ�������������������Ӧ�����ӷ���ʽ��
��2������ΪʲôҪ��0.13%I2���ȷ���Һ������ֱ����I2��ˮ��Һ��
��3��������Ʒ���������Ƶ������ٷֺ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com