
��2012��9��15�տ�ʼ��ȫ������������ر�����������ʾ����������⻪�ˣ�ͬ����飬ǿ��Ǵ���ձ�������Ϊ����¶�����Ա��ʵ�����Ŀ����������ʾ���㵺���й����ɷָ��������������ʾ��������ڻ�ѧ�仯����
A���ߺ��ںţ����㵺���й���!
B���߾��������Ǻ��죬�볪����
C�����������ձ���������Ļ���
D��������ɥʧ���ǵ���Υ������ͬ������ϵ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�ﰲ��ʡ�����и߶���ѧ�����л�ѧ�������Ծ��������棩 ���ͣ�ѡ����
���淴Ӧ��2NO2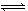 2NO+O2�ں��º����ܱ������з�Ӧ���ﵽƽ��״̬�ı�־��
2NO+O2�ں��º����ܱ������з�Ӧ���ﵽƽ��״̬�ı�־��
�ٵ�λʱ��������n molO2��ͬʱ����2n molNO2
�ڵ�λʱ��������n molO2 ��ͬʱ����2n mol NO
����NO2��NO��O2 �����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���ʵı�Ϊ2 : 2 : 1��״̬
�ܻ���������ɫ���ٸı��״̬
�ݻ��������ܶȲ��ٸı��״̬
��������ƽ����Է����������ٸı��״̬
A���٢ܢ� B���ڢۢ� C���٢ۢ� D���٢ڢۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�ﰲ��ʡ�����и�һ��һѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A��Ħ�������ʵ�������λ B��������Ħ��������2g
C��1molNH3��������17g D��1mol������ռ�����ԼΪ22.4L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�^����ʡ��һ��ѧ�ڵ�һ���ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
���ж��ڡ�Ħ������������ȷ����
A��Ħ���ǹ��ʿ�ѧ�罨����õ�һ��������
B��2H�ȿɱ�ʾ2����ԭ���ֿɱ�ʾ2 mol�����
C��1mol����6.02��1023��O2
D��Ħ�������ʵ����ĵ�λ�����Ħ������Ϊmol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�^����ʡ��һ��ѧ�ڵ�һ���ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
���������̨�������������ҹ���һЩ�ؽ����ж�γ��ִ�����������ʹ���ٹ�·�رգ�����ͣ�ɣ��������������ַ�ɢϵ
A.����Һ B.��Һ C.���� D.����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�^����ʡ�����и߶���һѧ���������ƻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��10�֣����³�ѹ�£�����1mol������ӻ�ѧ�������յ��������γ�1mol������ӻ�ѧ�����ų���������Ϊ���ܣ���λΪkJ.mol-1���������±��������ݣ�kJ��mol��1���ش����⣺
��ѧ�� | C��F | C��Cl | C��I | H��H | S��S | H��S |
���� | 427 | 330 | 218 | 436 | 255 | 339 |
��1���ɱ�������Ԥ��C��Br���ļ��ܷ�Χ��_________��C��Br���ܣ�_________���ش���ֵ�͵�λ��
��2���Ȼ�ѧ����ʽ2H2(g)��S2(g) = 2H2S(g)����H= QkJ��mol��1����Q=
��3����֪�����Ȼ�ѧ����ʽ��
��O2 (g) = O+2(g) + e- 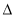 H1= +1175.7 kJ��mol-1 ��PtF6(g) + e- = PtF6��(g)
H1= +1175.7 kJ��mol-1 ��PtF6(g) + e- = PtF6��(g) 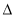 H2= -771.1 kJ��mol-1
H2= -771.1 kJ��mol-1
��O2+PtF6-(s) = O2+(g) + PtF6-(g)  H3= +482.2 kJ��mol-1
H3= +482.2 kJ��mol-1
��ӦO2(g) + (g) = O2+PtF6-(s) 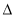 H=_____________ kJ��mol-1��
H=_____________ kJ��mol-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�^����ʡ�����и߶���һѧ���������ƻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ���¶��½�lmol CO(g)��lmol H2O(g)ͨ��һ���ݻ��̶����ܱ������з�Ӧ��CO(g) +H2O(g) CO2(g)+H2(g)���ﵽƽ���CO2�����ʵ���Ϊ0.6mol����ͨ��4molH2O��g�������ٴδﵽƽ���CO2�����ʵ���������
CO2(g)+H2(g)���ﵽƽ���CO2�����ʵ���Ϊ0.6mol����ͨ��4molH2O��g�������ٴδﵽƽ���CO2�����ʵ���������
A��n��CO2��=0.6 mol
B��n��CO2��=1 mol
C��1 mol��n��CO2����4 mol
D��0.6 mol��n��CO2����1 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�^����ʡ�߶���һѧ�����п����Ŀƻ�ѧ�Ծ��������棩 ���ͣ������
�˽�һЩ��ҩ��ʶ�����������ұ�������������ҩ�̼��þ����ù�ء���˾ƥ�֡�������������Ƽ
��1���������ڽ�����ʹ��ҩ����__________��
��2����ù�ص�������__________��
��3��ҩƬ���õ�����Ϊ�ϼ�������ˮ������ղ���Ľṹ��ʽ��______________��
��4��̼��þ������θ������ԭ����________________________�������ӷ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�^����ʡ�߶���ѧ�ڵ�һ�νβ��Ի�ѧ��B���Ծ��������棩 ���ͣ�ѡ����
�����ܱ������н��еķ�Ӧ��P��g��+ Q��g�� R��g��+ S��g������˵�����Գ��˵����һ��Ӧ�Ѿ��ﵽ��ѧƽ��״̬����
R��g��+ S��g������˵�����Գ��˵����һ��Ӧ�Ѿ��ﵽ��ѧƽ��״̬����
A��P��Q��R��S��Ũ�����
B��P��Q��R��S���ܱ������й���
C��P��Q��R��S��Ũ�Ȳ��ٱ仯
D����P��Ũ�ȱ�ʾ�Ļ�ѧ��Ӧ��������Q��Ũ�ȱ�ʾ�Ļ�ѧ��Ӧ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com