

 4N2(g)+6H2O(g) (��H <0)
4N2(g)+6H2O(g) (��H <0) 2N2(g)+6H2O(g)
2N2(g)+6H2O(g) 2N2O(g)+6H2O(g)
2N2O(g)+6H2O(g) 4N2O(g)+6H2O(g)
4N2O(g)+6H2O(g)
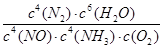 (2��) ��С(2��)
(2��) ��С(2��)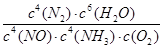 �����ڡ�H<0������Ӧ���ȣ�����ƽ�����ƣ�K��С�����������Ϣ��֪��Ӱ��N2O�����ʵ��������¶ȡ�n(NH3)/n(NO) ������Ũ�ȡ�
�����ڡ�H<0������Ӧ���ȣ�����ƽ�����ƣ�K��С�����������Ϣ��֪��Ӱ��N2O�����ʵ��������¶ȡ�n(NH3)/n(NO) ������Ũ�ȡ�

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 O2��g��=CO2��g���� ��H2��b kJ��mol��1
O2��g��=CO2��g���� ��H2��b kJ��mol��1 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g) ��H 1=��90.7 kJ��mol-1
CH3OH(g) ��H 1=��90.7 kJ��mol-1 CH3OCH3(g)+H2O(g) ��H 2=��23.5 kJ��mol-1
CH3OCH3(g)+H2O(g) ��H 2=��23.5 kJ��mol-1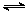 CO2(g)+H2(g) ��H 3=��41.2kJ��mol-1
CO2(g)+H2(g) ��H 3=��41.2kJ��mol-1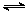 CH3OCH3(g)��CO2(g)�ġ�H�� kJ��mol-1��
CH3OCH3(g)��CO2(g)�ġ�H�� kJ��mol-1��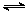 CH3OCH3(g)+H2O(g) ��H����CO��ƽ��ת�������¶ȡ�ѹǿ�仯��ϵ��ͼ1��ʾ������˵����ȷ���� ��
CH3OCH3(g)+H2O(g) ��H����CO��ƽ��ת�������¶ȡ�ѹǿ�仯��ϵ��ͼ1��ʾ������˵����ȷ���� ��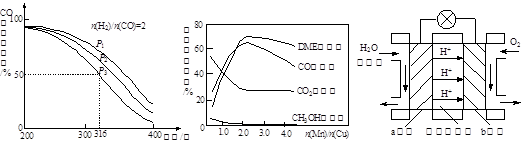
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����82kJ/mol | B����41kJ/mol | C����312kJ/mol | D����82kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 SO3��g����NO��g��
SO3��g����NO��g�� 2SO3��g�� ��H��a kJ��mol��1
2SO3��g�� ��H��a kJ��mol��1 2NO2��g�� ��H��b kJ��mol��1
2NO2��g�� ��H��b kJ��mol��1 SO3��g����NO��g�� ��H�� kJ��mol��1��
SO3��g����NO��g�� ��H�� kJ��mol��1�� SO3��g����NO��g����������ʵ�в���˵���÷�Ӧ�ﵽƽ��״̬���� ��ѡ����ţ���
SO3��g����NO��g����������ʵ�в���˵���÷�Ӧ�ﵽƽ��״̬���� ��ѡ����ţ��� CH3OH��g��������һ�ݻ��ɱ���ܱ������У�����10 mol CO��20 mol H2�����ںϳɼ״���CO��ƽ��ת���ʣ��������¶ȣ�T����ѹǿ��P���Ĺ�ϵ��ͼ��ʾ��
CH3OH��g��������һ�ݻ��ɱ���ܱ������У�����10 mol CO��20 mol H2�����ںϳɼ״���CO��ƽ��ת���ʣ��������¶ȣ�T����ѹǿ��P���Ĺ�ϵ��ͼ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��P��P���ļ��ܴ���P��Cl���ļ��� |
| B������Cl2(g)+ PCl3(g)��4PCl5(s)�ķ�Ӧ�ȡ�H |
| C��Cl��Cl���ļ���Ϊ(b��a+5��6c)/4 kJ?mol��1 |
| D��P��P���ļ���Ϊ(5a��3b+12c)/8 kJ?mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com