
Č”A”¢BĮ½·ŻĪļÖŹµÄĮæÅضČĻąµČµÄNaOHČÜŅŗ£¬Ģå»ż¾łĪŖ50mL£¬·Ö±šĻņĘäÖŠĶØČėŅ»¶ØĮæµÄCO2 ŗó£¬ŌŁ·Ö±šĻ”ŹĶĪŖ100mL”£
£Ø1£©ŌŚNaOHČÜŅŗÖŠĶØČėŅ»¶ØĮæµÄCO2ŗó£¬ČÜŅŗÖŠµÄČÜÖŹµÄ×é³ÉæÉÄÜŹĒ£ŗ
£Ø2£©ŌŚĻ”ŹĶŗóµÄČÜŅŗÖŠ·Ö±šÖšµĪ¼ÓČė0.1mol”¤L£1 µÄŃĪĖį£¬²śÉśµÄCO2µÄĢå»ż£Ø±ź×¼×“æö£©ÓėĖł¼ÓŃĪĖįµÄĢå»ż¹ŲĻµČēÓŅĶ¼ĖłŹ¾£ŗ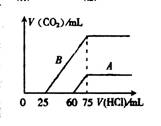
¢Ł ·Ö±š¼ÓČė×ćĮæµÄŃĪĖįŗó£¬ČÜŅŗÖŠµÄČÜÖŹŹĒ £¬
ŌNaOHČÜŅŗµÄĪļÖŹµÄĮæÅØ¶ČŹĒ ”£
¢Ś AĒśĻß±ķĆ÷£¬NaOHČÜŅŗĶØČėCO2ŗó£¬ĖłµĆČÜŅŗÖŠµÄČÜÖŹ
ŹĒ £¬ÓėŃĪĖį·“Ó¦²śÉśCO2µÄ×ī“óĢå»żŹĒ mL(±ź×¼×“æö)”£
¢Ū BĒśĻß±ķĆ÷£¬ŌNaOHČÜŅŗĶØČėCO2ŗó£¬ĖłµĆČÜÖŹµÄ»ÆѧŹ½ĪŖ £¬ ĘäĪļÖŹµÄĮæÖ®±ČĪŖ ”£
 æŚĖćĢāæØŗÓ±±ÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø
æŚĖćĢāæØŗÓ±±ÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø A¼Ó½šĢā ĻµĮŠ“š°ø
A¼Ó½šĢā ĻµĮŠ“š°ø Č«ÓŲāŹŌ¾ķĻµĮŠ“š°ø
Č«ÓŲāŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 °“ŅŖĒóĢīŠ“ĻĀĮŠæÕ°×£®
°“ŅŖĒóĢīŠ“ĻĀĮŠæÕ°×£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Č”A”¢BĮ½·ŻĪļÖŹµÄĮæÅضČĻąµČµÄNaOHČÜŅŗ£¬Ģå»ż¾łĪŖ50mL£¬·Ö±šĻņĘäÖŠĶØČėŅ»¶ØĮæµÄCO2 ŗó£¬ŌŁ·Ö±šĻ”ŹĶĪŖ100mL”£
£Ø1£©ŌŚNaOHČÜŅŗÖŠĶØČėŅ»¶ØĮæµÄCO2ŗó£¬ČÜŅŗÖŠµÄČÜÖŹµÄ×é³ÉæÉÄÜŹĒ£ŗ
£Ø2£©ŌŚĻ”ŹĶŗóµÄČÜŅŗÖŠ·Ö±šÖšµĪ¼ÓČė0.1mol”¤L£1 µÄŃĪĖį£¬²śÉśµÄCO2µÄĢå»ż£Ø±ź×¼×“æö£©ÓėĖł¼ÓŃĪĖįµÄĢå»ż¹ŲĻµČēÓŅĶ¼ĖłŹ¾£ŗ
¢Ł ·Ö±š¼ÓČė×ćĮæµÄŃĪĖįŗó£¬ČÜŅŗÖŠµÄČÜÖŹŹĒ £¬
ŌNaOHČÜŅŗµÄĪļÖŹµÄĮæÅØ¶ČŹĒ ”£
¢Ś AĒśĻß±ķĆ÷£¬NaOHČÜŅŗĶØČėCO2ŗó£¬ĖłµĆČÜŅŗÖŠµÄČÜÖŹ
ŹĒ £¬ÓėŃĪĖį·“Ó¦²śÉśCO2µÄ×ī“óĢå»żŹĒ mL(±ź×¼×“æö)”£
¢Ū BĒśĻß±ķĆ÷£¬ŌNaOHČÜŅŗĶØČėCO2ŗó£¬ĖłµĆČÜÖŹµÄ»ÆѧŹ½ĪŖ £¬ ĘäĪļÖŹµÄĮæÖ®±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗŚĮś½Ź”ŗ×øŚŅ»ÖŠø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĪŹ“šĢā
Č”A”¢BĮ½·ŻĪļÖŹµÄĮæÅضČĻąµČµÄNaOHČÜŅŗ£¬Ģå»ż¾łĪŖ50 mL£¬·Ö±šĻņĘäÖŠĶØČėŅ»¶ØĮæµÄCO2ŗó£¬ŌŁ·Ö±šĻ”ŹĶĪŖ100 mL”£
(1)ŌŚNaOHČÜŅŗÖŠĶØČėŅ»¶ØĮæµÄCO2ŗó£¬ČÜŅŗÖŠČÜÖŹµÄ×é³ÉæÉÄÜŹĒ£ŗ
¢Ł________£»¢Ś________£»¢Ū________£»¢Ü________”£
(2)ŌŚĻ”ŹĶŗóµÄČÜŅŗÖŠ·Ö±šÖšµĪ¼Ó0.1 mol/LµÄŃĪĖį£¬²śÉśµÄCO2µÄĢå»ż(±ź×¼×“æö)
ÓėĖł¼ÓŃĪĖįµÄĢå»ż¹ŲĻµČēĶ¼ĖłŹ¾£ŗ
¢Ł·Ö±š¼ÓČė×ćĮæµÄŃĪĖįŗóµÄČÜŅŗÖŠµÄČÜÖŹŹĒ________£¬ŌNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ________”£
¢ŚAĒśĻß±ķĆ÷£¬ŌČÜŅŗĶØČėCO2ŗó£¬ĖłµĆČÜÖŹÓėHCl·“Ó¦²śÉśCO2µÄ×ī“óĢå»żŹĒ________mL(±ź×¼×“æö)”£
¢ŪBĒśĻß±ķĆ÷£¬ŌČÜŅŗĶØČėCO2ŗó£¬ĖłµĆČÜÖŹµÄ»ÆѧŹ½ĪŖ________£¬ĘäĪļÖŹµÄĮæÖ®±ČĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğĢģ½ņŹŠøßČżµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
Č”A”¢BĮ½·ŻĪļÖŹµÄĮæÅضČĻąµČµÄNaOHČÜŅŗ£¬Ģå»ż¾łĪŖ50mL£¬·Ö±šĻņĘäÖŠĶØČėŅ»¶ØĮæµÄCO2 ŗó£¬ŌŁ·Ö±šĻ”ŹĶĪŖ100mL”£
£Ø1£©ŌŚNaOHČÜŅŗÖŠĶØČėŅ»¶ØĮæµÄCO2ŗó£¬ČÜŅŗÖŠµÄČÜÖŹµÄ×é³ÉæÉÄÜŹĒ£ŗ
£Ø2£©ŌŚĻ”ŹĶŗóµÄČÜŅŗÖŠ·Ö±šÖšµĪ¼ÓČė0.1mol”¤L£1 µÄŃĪĖį£¬²śÉśµÄCO2µÄĢå»ż£Ø±ź×¼×“æö£©ÓėĖł¼ÓŃĪĖįµÄĢå»ż¹ŲĻµČēÓŅĶ¼ĖłŹ¾£ŗ
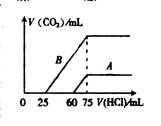
¢Ł ·Ö±š¼ÓČė×ćĮæµÄŃĪĖįŗó£¬ČÜŅŗÖŠµÄČÜÖŹŹĒ £¬
ŌNaOHČÜŅŗµÄĪļÖŹµÄĮæÅØ¶ČŹĒ ”£
¢Ś AĒśĻß±ķĆ÷£¬NaOHČÜŅŗĶØČėCO2ŗó£¬ĖłµĆČÜŅŗÖŠµÄČÜÖŹ
ŹĒ £¬ÓėŃĪĖį·“Ó¦²śÉśCO2µÄ×ī“óĢå»żŹĒ mL(±ź×¼×“æö)”£
¢Ū BĒśĻß±ķĆ÷£¬ŌNaOHČÜŅŗĶØČėCO2ŗó£¬ĖłµĆČÜÖŹµÄ»ÆѧŹ½ĪŖ £¬ ĘäĪļÖŹµÄĮæÖ®±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com