

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 | 1 | 2 | 3 | 4 |
| ��������������Һ�����/mL | 25 | 25 | 25 | 25 |
| ���ɳ�����������/g | 0.29 | X | 0.87 | 0.87 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
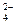 ��CO
��CO ��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش�
��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
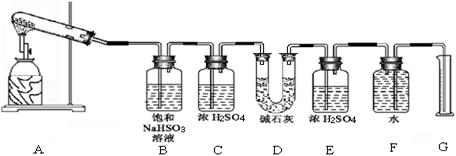
 �������ϴ����������� ��
�������ϴ����������� ��| ʵ��С�� | ��ȡCaSO4 ������(g) | װ��D���� ������(g) | ��ȡ���������װ�ò������������ (����ɱ�״������������) (mL) |
| һ | 4.08 | 2. 56 56 | 224 |
| �� | 5.44 | 2.56 | 448 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
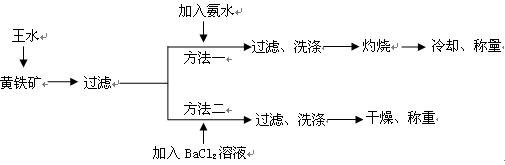
 ��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�
��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�| A��BaCl2 | B��NaOH | C��Na2SO4 | D��HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

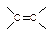 ��IBr ��
��IBr ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ��
��
A��װ�â���һ��ʵ��������װ�ã����ڷ�����������ռ�����,��ͭм��ϡ���� |
B��װ�â��У�aΪ������dΪ���� |
C��װ�âۿ������ռ�H2��NH3��Cl2��HCl��NO2�� |
D��װ�â������ڲ������������װ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 1��ʵ������ܴﵽʵ��Ŀ�ĵ��� �� ��
1��ʵ������ܴﵽʵ��Ŀ�ĵ��� �� ��| | ʵ����� | ʵ��Ŀ�� |
| A | ����������ϩ�ֱ�ͨ�����Ը��������Һ�� | ���Լ����������ϩ |
| B | ������Һ������ϡ���ᣬ�ټ�������������ͭ������ | ȷ�������Ƿ�ˮ�� |
| C | ��ƾ��Ӵ���Ļ��Һ�м�������� | ȷ���ƾ��л��д��� |
| D | ���м������Ը��������Һ | ��֤���ṹ�д���̼̼˫�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com