
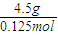 =36g/mol£¬ĖłŅŌAµÄĻą¶Ō·Ö×ÓÖŹĮæ²»»į“óÓŚ36£¬ĖłŅŌŅ»¶Ø²»ŗ¬ĮņŌŖĖŲ£¬×é³ÉŌŖĖŲĪŖC”¢H”¢FČżÖÖ£¬Ęä·Ö×ÓŹ½ĪŖCH3F£¬¹Ź“š°øĪŖ£ŗ36£»C”¢H”¢F£»CH3F£»
=36g/mol£¬ĖłŅŌAµÄĻą¶Ō·Ö×ÓÖŹĮæ²»»į“óÓŚ36£¬ĖłŅŌŅ»¶Ø²»ŗ¬ĮņŌŖĖŲ£¬×é³ÉŌŖĖŲĪŖC”¢H”¢FČżÖÖ£¬Ęä·Ö×ÓŹ½ĪŖCH3F£¬¹Ź“š°øĪŖ£ŗ36£»C”¢H”¢F£»CH3F£»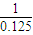 =500KJµÄČČĮ棬¼“2CH3F£Øg£©+3O2£Øg£©=2CO2£Øg£©+2HF£Øg£©+2H2O£Ø1£©£»”÷H=-1000kJ?mol-1£¬¹Ź“š°øĪŖ£ŗ2CH3F£Øg£©+3O2£Øg£©=2CO2£Øg£©+2HF£Øg£©+2H2O£Ø1£©£»”÷H=-1000kJ?mol-1£®
=500KJµÄČČĮ棬¼“2CH3F£Øg£©+3O2£Øg£©=2CO2£Øg£©+2HF£Øg£©+2H2O£Ø1£©£»”÷H=-1000kJ?mol-1£¬¹Ź“š°øĪŖ£ŗ2CH3F£Øg£©+3O2£Øg£©=2CO2£Øg£©+2HF£Øg£©+2H2O£Ø1£©£»”÷H=-1000kJ?mol-1£®

 æŚĖćÄÜŹÖĻµĮŠ“š°ø
æŚĖćÄÜŹÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĘųĢåAÓÉC”¢H”¢S”¢FÖŠµÄČżÖÖŌŖĖŲ×é³É£¬Č”±ź×¼×“æöĻĀµÄ1.12 LĘųĢåA×°ČėŅ»øö±”ĤĄļ£¬“üŗĶĘųĢåµÄ×ÜÖŹĮæĪŖ2.20 g(²»æ¼ĀĒø”Į¦)”£øł¾ŻÉĻŹöŹż¾ŻĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)¹ĄĖćAµÄĻą¶Ō·Ö×ÓÖŹĮæ²»»į“óÓŚ__________”£
(2)øĆĘųĢåAÖŠŗ¬²»ŗ¬SŌŖĖŲ£æ__________”£
(3)Č·¶ØAµÄ»ÆѧŹ½ĪŖ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com