
| �� | �� | �� | �� | |
| ���ȼ�����/g | 5g | 10g | 15g | 20g |
| �������/L | 2.24L | 4.48L | 6.72L | 6.72L |
 =0.3mol����
=0.3mol����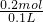 =2mol/L��
=2mol/L��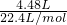 =0.2mol
=0.2mol =
= mol����m��Al��=
mol����m��Al��= mol��27g/mol=3.6g��
mol��27g/mol=3.6g�� ��100%=36%��
��100%=36%�� =0.267mol��
=0.267mol�� =0.08mol��
=0.08mol�� 2Fe+Al2O3��֪��0.08molFe2O3��ȫ��Ӧ��Ҫ0.16molAl����Al������Fe2O3��ȫ��Ӧ������Ԫ���غ��֪n��Fe��=2n��Fe2O3��=0.16mol����m��Fe��=0.16mol��56g/mol=8.96g��
2Fe+Al2O3��֪��0.08molFe2O3��ȫ��Ӧ��Ҫ0.16molAl����Al������Fe2O3��ȫ��Ӧ������Ԫ���غ��֪n��Fe��=2n��Fe2O3��=0.16mol����m��Fe��=0.16mol��56g/mol=8.96g��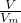 �������������ʵ������ٸ��ݷ���ʽ����n��NaOH��������c=
�������������ʵ������ٸ��ݷ���ʽ����n��NaOH��������c= ��������������Һ�����ʵ���Ũ�ȣ�
��������������Һ�����ʵ���Ũ�ȣ�

 ȫ��������ϵ�д�
ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�������й�ʵ���˵������������۲���ȷ���� ������ţ�
A���Ʊ�Fe(OH)3����ʱ��Ӧ����ˮ�еμӱ���FeCl3 ��Һ�����������ȵ���Һ�ʺ��ɫΪֹ��
B���ⶨ�к���ʵ���У�ÿ��ʵ���Ӧ���������¶ȣ���������ʼ�¶ȣ�NaOH��Һ����ʼ�¶Ⱥͷ�Ӧ����Һ������¶ȡ�
C����һ�����ʵ�����Һ���ƵĹ����У�û��ϴ���ձ��Ͳ�����������ʱ��ˮ�����˿̶��ߡ�����ƿû�и������ʹ������ҺŨ��ƫ�͡�
D����FeCl3��Һ�е���KI��������Һ����Һ����ɫ��
E�����ۺ�����þ����������ĩ��ϣ����¾��ܷ������ȷ�Ӧ��
F����֤AgNO3��Һ���Ƿ����Al(NO3)3���ɼ��������ˮ�����а�ɫ�����������Al(NO3)3
G��ϡ��Ũ����ʱ��Ҫ��ˮ�������ڻ���ע��Ũ�����У����ò��������Ͻ��衣
��2��ʵ��������500ml 0.1 mol/L��Na2CO3��Һ������������ƽ��ȡ̼���Ʒ�ĩ g��
����ʱӦѡ�õ������У�������ƽ��ҩ�ס��ձ�����Ͳ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�������й�ʵ���˵������������۲���ȷ���� ������ţ�
A���Ʊ�Fe(OH)3����ʱ��Ӧ����ˮ�еμӱ���FeCl3��Һ�����������ȵ���Һ�ʺ��ɫΪֹ��
B���ⶨ�к���ʵ���У�ÿ��ʵ���Ӧ���������¶ȣ���������ʼ�¶ȣ�NaOH��Һ����ʼ�¶Ⱥͷ�Ӧ����Һ������¶ȡ�
C����һ�����ʵ�����Һ���ƵĹ����У�û��ϴ���ձ��Ͳ�����������ʱ��ˮ�����˿̶��ߡ�����ƿû�и������ʹ������ҺŨ��ƫ�͡�
D����FeCl3��Һ�е���KI��������Һ����Һ����ɫ��
E�����ۺ�����þ����������ĩ��ϣ����¾��ܷ������ȷ�Ӧ��
F����֤AgNO3��Һ���Ƿ����Al(NO3)3���ɼ��������ˮ�����а�ɫ�����������Al(NO3)3
G��ϡ��Ũ����ʱ��Ҫ��ˮ�������ڻ���ע��Ũ�����У����ò��������Ͻ��衣
��2��ʵ��������500ml 0.1 mol/L��Na2CO3��Һ������������ƽ��ȡ̼���Ʒ�ĩ g��
����ʱӦѡ�õ������У�������ƽ��ҩ�ס��ձ�����Ͳ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����������ʵ����ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��1�������й�ʵ���˵������������۲���ȷ���� ������ţ�
| A���Ʊ�Fe(OH)3����ʱ��Ӧ����ˮ�еμӱ���FeCl3��Һ�����������ȵ���Һ�ʺ��ɫΪֹ�� |
| B���ⶨ�к���ʵ���У�ÿ��ʵ���Ӧ���������¶ȣ���������ʼ�¶ȣ�NaOH��Һ����ʼ�¶Ⱥͷ�Ӧ����Һ������¶ȡ� |
| C����һ�����ʵ�����Һ���ƵĹ����У�û��ϴ���ձ��Ͳ�����������ʱ��ˮ�����˿̶��ߡ�����ƿû�и������ʹ������ҺŨ��ƫ�͡� |
| D����FeCl3��Һ�е���KI��������Һ����Һ����ɫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����������ʵ����ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��1�������й�ʵ���˵������������۲���ȷ���� ������ţ�
A���Ʊ�Fe(OH)3����ʱ��Ӧ����ˮ�еμӱ���FeCl3 ��Һ�����������ȵ���Һ�ʺ��ɫΪֹ��
B���ⶨ�к���ʵ���У�ÿ��ʵ���Ӧ���������¶ȣ���������ʼ�¶ȣ�NaOH��Һ����ʼ�¶Ⱥͷ�Ӧ����Һ������¶ȡ�
C����һ�����ʵ�����Һ���ƵĹ����У�û��ϴ���ձ��Ͳ�����������ʱ��ˮ�����˿̶��ߡ�����ƿû�и������ʹ������ҺŨ��ƫ�͡�
D����FeCl3��Һ�е���KI��������Һ����Һ����ɫ��
E�����ۺ�����þ����������ĩ��ϣ����¾��ܷ������ȷ�Ӧ��
F����֤AgNO3��Һ���Ƿ����Al(NO3)3���ɼ��������ˮ�����а�ɫ�����������Al(NO3)3
G��ϡ��Ũ����ʱ��Ҫ��ˮ�������ڻ���ע��Ũ�����У����ò��������Ͻ��衣
��2��ʵ��������500ml 0.1 mol/L��Na2CO3��Һ������������ƽ��ȡ̼���Ʒ�ĩ g��
����ʱӦѡ�õ������У�������ƽ��ҩ�ס��ձ�����Ͳ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com