
ĻĀĮŠø÷×鏿¾ŻÖŠ£¬Ē°ÕßøÕŗĆŹĒŗóÕßĮ½±¶µÄŹĒ (”””” )
A£®2 molĖ®µÄĦ¶ūÖŹĮæŗĶ1 molĖ®µÄĦ¶ūÖŹĮæ
B£®200 mL 1 mol/LĀČ»ÆøĘČÜŅŗÖŠc(Cl£)ŗĶ100 mL 2 mol/LĀČ»Æ¼ŲČÜŅŗÖŠc(Cl£)
C£®64 g¶žŃõ»ÆĮņÖŠŃõŌ×ÓŹżŗĶ±ź×¼×“æöĻĀ22.4 LŅ»Ńõ»ÆĢ¼ÖŠŃõŌ×ÓŹż
D£®20%NaOHČÜŅŗÖŠNaOHµÄĪļÖŹµÄĮæÅضČŗĶ10%NaOHČÜŅŗÖŠNaOHµÄĪļÖŹµÄĮæÅضČ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĀĮÓĆĶ¾¹ć·ŗ£¬ÓĆĀĮĶĮæó(Ö÷ŅŖ³É·ÖĪŖAl2O3·nH2O”¢ÉŁĮæSiO2ŗĶFe2O3)ÖĘČ”AlÓŠČēĻĀĶ¾¾¶£ŗ
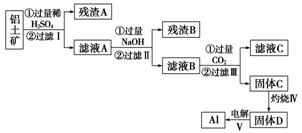
(1)ĀĖŅŗAæÉÓĆÓŚ¾»Ė®£¬Ęä¾»Ė®ŌĄķÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ĪŖ________________________”£
(2)×ĘÉÕŹ±Ź¢·ÅŅ©Ę·µÄŅĒĘ÷Ćū³ĘŹĒ__________”£
(3)²½Öč¢õÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____________________________________”£
(4)²½Öč¢óÖŠÉś³É¹ĢĢåC·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________________”£
(5)Č”ĀĖŅŗB 100 mL£¬¼ÓČė1 mol·L£1ŃĪĖį200 mL£¬³ĮµķĮæ“ļµ½×ī“óĒŅÖŹĮæĪŖ11.7 g”£ŌņĀĖŅŗBÖŠc(AlO )£½______£¬c(Na£«)______(Ģī”°>”±”¢”°£½”±»ņ”°<”±)2 mol·L£1”£
)£½______£¬c(Na£«)______(Ģī”°>”±”¢”°£½”±»ņ”°<”±)2 mol·L£1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
·“Ó¦ A£«B”Ŗ”śC(¦¤H£¼0)·ÖĮ½²½½ųŠŠ£ŗ¢ŁA£«B”Ŗ”śX (¦¤H £¾0)£¬¢ŚX”Ŗ”śC(¦¤H£¼0)”£ĻĀĮŠŹ¾ŅāĶ¼ÖŠ£¬ÄÜÕżČ·±ķŹ¾×Ü·“Ó¦¹ż³ĢÖŠÄÜĮæ±ä»ÆµÄŹĒ(””””)
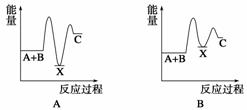
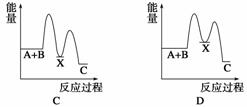
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ£ŗHCN(aq)ÓėNaOH(aq)·“Ó¦µÄ¦¤H£½£12.1 kJ·mol£1£»HCl(aq)ÓėNaOH(aq)·“Ó¦µÄ¦¤H£½£55.6 kJ·mol£1£¬ŌņHCNŌŚĖ®ČÜŅŗÖŠµēĄėµÄ¦¤HµČÓŚ(””””)
A£®£67.7 kJ·mol£1 B£®£43.5 kJ·mol£1
C£®£«43.5 kJ·mol£1 D£®£«67.7 kJ·mol£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½ŗĢåĒų±š×ĒŅŗŗĶČÜŅŗµÄ±¾ÖŹŹĒ £Ø £©
A”¢ ¶”“ļ¶ūŠ§Ó¦ B”¢·ÖÉ¢ÖŹĮ£×ÓµÄÖ±¾¶“óŠ”
C”¢ æÉŅŌĶø¹żĀĖÖ½ D”¢ÄÜ·¢Éś¾Ū³Į
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖI2+SO32-+H2O===SO42-+2I-+2H+”£Ä³ĪŽÉ«ČÜŅŗÖŠÖ»æÉÄÜŗ¬ÓŠI-£¬NH4+,Ba2+, SO32-,MnO4-ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ£¬ČōĻņøĆČÜŅŗÖŠµĪ¼ÓÉŁĮæµÄäåĖ®£¬ČÜŅŗČŌĪŖĪŽÉ«£¬ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ ( )
A.øĆČÜŅŗÖŠæĻ¶Ø²»ŗ¬I- B. øĆČÜŅŗÖŠæÉÄÜŗ¬ÓŠBa2+
C. øĆČÜŅŗÖŠæĻ¶Øŗ¬ÓŠNH4+ D. øĆČÜŅŗÖŠæÉÄÜŗ¬ÓŠMnO4-
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
·ŪĩדŹŌŃłAŹĒÓɵČĪļÖŹµÄĮæµÄMgOŗĶFe2O3×é³ÉµÄ»ģŗĻĪļ”£½ųŠŠČēĻĀŹµŃé£ŗ
¢ŁČ”ŹŹĮæA½ųŠŠĀĮČČ·“Ó¦£¬²śĪļÖŠÓŠµ„ÖŹBÉś³É”£(ĢįŹ¾£ŗĀĮÓėŃõ»ÆĢś·¢ÉśµÄ·“Ó¦³ĘĪŖĀĮČČ·“Ó¦)
¢ŚĮķČ”20g AČ«²æČÜÓŚ0.15L 6.0mol”¤L£1ŃĪĖįÖŠ£¬µĆČÜŅŗC”£
¢Ū½«¢ŁÖŠµĆµ½µÄµ„ÖŹBŗĶČÜŅŗC·“Ó¦£¬·Å³öĘųĢå1.12L(±ź×¼×“æöĻĀ)£¬Ķ¬Ź±Éś³ÉČÜŅŗD£¬»¹²ŠĮō¹ĢĢåB”£
¢ÜÓĆKSCNČÜŅŗ¼ģŃ鏱£¬ČÜŅŗD²»±äÉ«”£
(1)·“Ó¦¢ŁµÄ²śĪļÖŠµÄµ„ÖŹBŹĒ________”£
(2)¢ŚĖł·¢Éśø÷·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ
_____________________________________________________________ӣ
(3)ČōČÜŅŗDµÄĢå»żČŌĪŖ0.15L£¬ŌņøĆČÜŅŗÖŠ
c(Mg2£«)ĪŖ________ £¬c(Fe2£«)ĪŖ________£Ø±£ĮōŠ”ŹżµćŗóĮ½Ī»£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄūĆŹĻ©ŹĒŅ»ÖÖŹ³ÓĆĻćĮĻ£¬Ęä½į¹¹¼ņŹ½ČēĶ¼£¬ÓŠ¹ŲÄūĆŹĻ©µÄ·ÖĪö“ķĪóµÄŹĒ
A£®ŌŚŅ»¶ØĢõ¼žĻĀ£¬1molÄūĆŹĻ©æÉÓė2molH2ĶźČ«¼Ó³É
B£®ÄūĆŹĻ©µÄŅ»ĀČ“śĪļÓŠ7ÖÖ
C£®ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÄūĆŹĻ©æÉ·¢Éś¼Ó³É”¢Č”“ś”¢Ńõ»Æ”¢»¹Ō·“Ó¦
D£®ÄūĆŹĻ©·Ö×ÓÖŠĖłÓŠĢ¼Ō×Ó²»æÉÄܶ¼ŌŚĶ¬Ņ»Ę½Ćę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠŅ»Ęæ³ĪĒåČÜŅŗ£¬ĘäÖŠæÉÄÜŗ¬ÓŠNH ”¢K£«”¢Na£«”¢Mg2£«”¢Ba2£«”¢Al3£«”¢Fe3£«”¢Cl£”¢I£”¢NO
”¢K£«”¢Na£«”¢Mg2£«”¢Ba2£«”¢Al3£«”¢Fe3£«”¢Cl£”¢I£”¢NO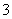 ”¢CO
ӢCO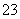 ӢSO
”¢SO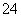 ÖŠµÄ¼øÖÖ”£Č”øĆČÜŅŗ½ųŠŠŅŌĻĀŹµŃ锣
ÖŠµÄ¼øÖÖ”£Č”øĆČÜŅŗ½ųŠŠŅŌĻĀŹµŃ锣
(1)ĢīŠ“±ķÖŠæÕ°×£ŗ
| ŹµŃé²½Öč | æĻ¶Ø²»“ęŌŚµÄĄė×Ó |
| ¢ŁÓĆpHŹŌÖ½¼ģŃ飬ČÜŅŗ³ŹĒæĖįŠŌ | |
| ¢ŚČ”³ö²æ·ÖČÜŅŗ£¬¼ÓČėÉŁĮæCCl4¼°ŹżµĪŠĀÖʵÄĀČĖ®£¬¾Õńµ“”¢¾²ÖĆŗóCCl4²ć³Ź×ĻŗģÉ« | |
| ¢ŪĮķČ”²æ·ÖČÜŅŗ£¬ĻņĘäÖŠ¼ÓČėNaOHČÜŅŗ£¬Ź¹ČÜŅŗ“ÓĖįŠŌÖš½„±äĪŖ¼īŠŌ£¬ŌŚµĪ¼Ó¹ż³ĢÖŠ¼°µĪ¼ÓĶź±Ļŗ󣬾łĪŽ³Įµķ²śÉś | |
| ¢ÜČ”¢ŪÖŠµÄ²æ·Ö¼īŠŌČÜŅŗ¼ÓČČ£¬ÓŠĘųĢå·Å³ö£¬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶ | |
| ¢ŻĮķČ”¢ŪÖŠµÄ²æ·Ö¼īŠŌČÜŅŗ£¬ĻņĘäÖŠ¼ÓČėNa2CO3ČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś |
(2)øł¾ŻŅŌÉĻŹĀŹµ£¬øĆČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄĄė×ÓŹĒ________”£
(3)Š“³öŹµŃé¢Ś”¢¢Ü”¢¢ŻÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
ŹµŃé¢Ś________________________________________________________________________£»
ŹµŃé¢Ü________________________________________________________________________£»
ŹµŃé¢Ż________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com