
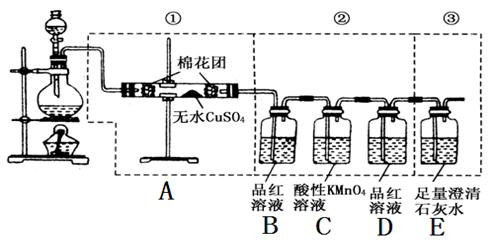
 CO2ӟ+2SO2ӟ+2H2O
CO2”ü+2SO2”ü+2H2O CO2”ü+2SO2”ü+2H2O”£²śĪļÓŠČżÖÖĖ®ÕōĘų”¢CO2ŗĶSO2£¬ŃéÖ¤Ė®ÕōĘųÓĆĪŽĖ®ĮņĖįĶ£¬CO2ÓƱ„ŗĶŹÆ»ŅĖ®£¬SO2ÓĆĘ·ŗģČÜŅŗ”£ÓÉÓŚŗóĮ½Õ߶¼ŠčŅŖĶعżČÜŅŗ£¬Ņņ“ĖŹ×ĻČŃéÖ¤Ė®ÕōĘų”£ŅņĪŖSO2Ņ²æÉŅŌŹ¹ŹÆ»ŅĖ®±ä»ģ×Ē£¬Ņņ“ĖŌŚ¼ģŃéCO2Ö®Ē°£¬ŠčŅŖĻČ¼ģŃéSO2£¬²¢ĒŅ»¹ŅŖĶźČ«³żČ„SO2£¬ŅŌ±ÜĆāøÉČÅCO2µÄ¼ģŃ锣
CO2”ü+2SO2”ü+2H2O”£²śĪļÓŠČżÖÖĖ®ÕōĘų”¢CO2ŗĶSO2£¬ŃéÖ¤Ė®ÕōĘųÓĆĪŽĖ®ĮņĖįĶ£¬CO2ÓƱ„ŗĶŹÆ»ŅĖ®£¬SO2ÓĆĘ·ŗģČÜŅŗ”£ÓÉÓŚŗóĮ½Õ߶¼ŠčŅŖĶعżČÜŅŗ£¬Ņņ“ĖŹ×ĻČŃéÖ¤Ė®ÕōĘų”£ŅņĪŖSO2Ņ²æÉŅŌŹ¹ŹÆ»ŅĖ®±ä»ģ×Ē£¬Ņņ“ĖŌŚ¼ģŃéCO2Ö®Ē°£¬ŠčŅŖĻČ¼ģŃéSO2£¬²¢ĒŅ»¹ŅŖĶźČ«³żČ„SO2£¬ŅŌ±ÜĆāøÉČÅCO2µÄ¼ģŃ锣

 ÄĻ“ó½ĢøØĒĄĻČĘšÅÜŹī¼ŁĻĪ½Ó½Ģ³ĢÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
ÄĻ“ó½ĢøØĒĄĻČĘšÅÜŹī¼ŁĻĪ½Ó½Ģ³ĢÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®CuŗĶĻ”ĻõĖį·“Ó¦ | B£®ZnŗĶĻ”ŃĪĖį·“Ó¦ | C£®FeŗĶĻ”ĮņĖį·“Ó¦ | D£®A1ŗĶĻ”ŃĪĖį·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
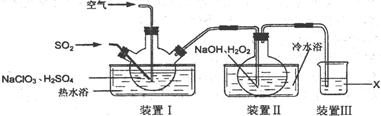
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
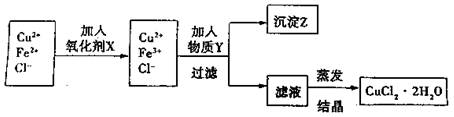
| | ĒāŃõ»ÆĪļæŖ Ź¼³ĮµķŹ±µÄpH Ź¼³ĮµķŹ±µÄpH | ĒāŃõ»ÆĪļ³ĮµķĶźČ«Ź±µÄpH |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |
 ”££ØĢīŠņŗÅ£©
”££ØĢīŠņŗÅ£© ”£
”£ ++H2”üÉč¼ĘŹµŃ饓ÖĘČ”CuCl2ČÜŅŗ£¬øĆĶ¬Ń§Éč¼ĘµÄ×°ÖĆÓ¦øĆĪŖ ”££ØĢī”°Ōµē³Ų”±»ņ”°µē½ā³Ų”±£©
++H2”üÉč¼ĘŹµŃ饓ÖĘČ”CuCl2ČÜŅŗ£¬øĆĶ¬Ń§Éč¼ĘµÄ×°ÖĆÓ¦øĆĪŖ ”££ØĢī”°Ōµē³Ų”±»ņ”°µē½ā³Ų”±£©
| ĪļÖŹ | FeS | MnS | CuS | PbS | HgS | ZnS |
| Ksp | 6.3”Į10-18 | 2.5”Į-13 | 1.3”Į10-36 | 3.4”Į10-28 | 6.4”Į10-53 | 1.6”Į10-24 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
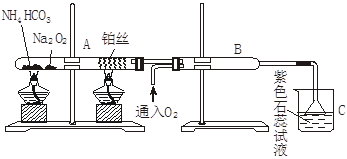
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
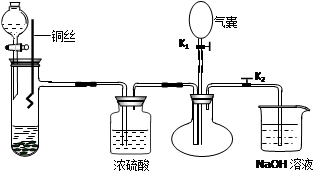
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā


 ĀĖŅŗ
ĀĖŅŗ
 ¾«ŃĪ
¾«ŃĪ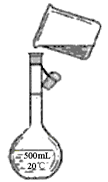
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
| ŹµŃé²½Öč | Ō¤ĘŚĻÖĻóÓė½įĀŪ |
| ²½Öč1£ŗ | |
| ²½Öč2£ŗ | |
| ²½Öč3£ŗ | |
| ”” | |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com