
���������ʣ�������Ũ���ᡢ�����������⡢ͭƬ���ơ���̿������
��ѡ����ʵ�������գ����ش��й����⣺
��1������Ͷ����з�̪��ˮ�У����������ɡ���Һ��죬��д���÷�Ӧ�����ӷ���ʽ��
��2������������������Ե�ʵ�飬ѡ����Լ�Ӧ�Ƕ��������
��3����ʹ���Ը��������Һ��ɫ������������Ϊ
��4���������ʼ䷴Ӧ�����ɵ������������ʹ����ʯ��ˮ����ǣ���д���÷�Ӧ�Ļ�ѧ����ʽ��
��5������������(���������е�һ��)�Ļ�ѧ����ʽ
����8�֣� ��1��2Na ��2H2O ===2Na+ + 2OH�� + H2 �� ��2�֣�
��2������(H2S ) ��1�֣� ��3����������SO2 ) (1��)
��4��C+2H2SO4��Ũ�� 2SO2��+CO2��+2
H2O��2�֣�����Ũ����δд��1�֣�
��5�� 4NH3��5O2
2SO2��+CO2��+2
H2O��2�֣�����Ũ����δд��1�֣�
��5�� 4NH3��5O2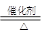 4NO��6H2O ��2�֣�
4NO��6H2O ��2�֣�
��������
�����������1������ͭƬ���ơ���̿�У�ֻ�����ܺ�ˮ��Ӧ�����ӷ���ʽΪ2Na ��2H2O ===2Na+ + 2OH�� + H2 ����
��2��ѡ��S����ͼ�̬��������(H2S )��
��3�������������Ҿ��л�ԭ�Ե��Ƕ�������SO2 )��
��4��ʹ����ʯ��ˮ����ǵ�������SO2��CO2����C��Ũ���ᷴӦ������ʽΪC+2H2SO4��Ũ�� 2SO2��+CO2��+2
H2O��
2SO2��+CO2��+2
H2O��
��5�����������ǰ��Ĵ������������������ڴ��������·����ķ�Ӧ������ʽʽ��4NH3��5O2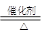 4NO��6H2O��
4NO��6H2O��
���㣺��������� ���ӷ���ʽ����д ��ѧ����ʽ����д
���������⿼�������������ʡ����ӷ���ʽ����д�ͻ�ѧ����ʽ����д�����֪ʶ����Ŀ�ѶȲ���ע�����֪ʶ�����ա�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�����м�����ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
���������ʣ�������Ũ���ᡢ�����������⡢ͭƬ���ơ���̿������
��ѡ����ʵ�������գ����ش��й����⣺
��1������Ͷ����з�̪��ˮ�У����������ɡ���Һ��죬��д���÷�Ӧ�����ӷ���ʽ��
��2������������������Ե�ʵ�飬ѡ����Լ�Ӧ�Ƕ��������
��3����ʹ���Ը��������Һ��ɫ������������Ϊ
��4���������ʼ䷴Ӧ�����ɵ������������ʹ����ʯ��ˮ����ǣ���д���÷�Ӧ�Ļ�ѧ����ʽ��
��5������������(���������е�һ��)�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ����һ�С����и�һ��ѧ����ĩ������ѧ�Ծ����������� ���ͣ������
���������ʣ�������Ũ���ᡢ�����������⡢ͭƬ���ơ���̿������
��ѡ����ʵ�������գ����ش��й����⣺
��1������Ͷ����з�̪��ˮ�У����������ɡ���Һ��죬��д���÷�Ӧ�����ӷ���
ʽ��
��2������������������Ե�ʵ�飬ѡ����Լ�Ӧ�Ƕ��������
��3����ʹ���Ը��������Һ��ɫ������������Ϊ
��4���������ʼ䷴Ӧ�����ɵ������������ʹ����ʯ��ˮ����ǣ���д���÷�Ӧ�Ļ�ѧ����ʽ��
��5������������(���������е�һ��)�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ�����и�һ��ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ������
���������ʣ�������Ũ���ᡢ�����������⡢ͭƬ���ơ���̿������
��ѡ����ʵ�������գ����ش��й����⣺
��1������Ͷ����з�̪��ˮ�У����������ɡ���Һ��죬��д���÷�Ӧ�����ӷ���
ʽ��
��2������������������Ե�ʵ�飬ѡ����Լ�Ӧ�Ƕ��������
��3����ʹ���Ը��������Һ��ɫ������������Ϊ
��4���������ʼ䷴Ӧ�����ɵ������������ʹ����ʯ��ˮ����ǣ���д���÷�Ӧ�Ļ�ѧ����ʽ��
��5������������(���������е�һ��)�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ʣ�������Ũ���ᡢ�����������⡢ͭƬ���ơ���̿������
��ѡ����ʵ�������գ����ش��й����⣺
��1������Ͷ����з�̪��ˮ�У����������ɡ���Һ��죬��д���÷�Ӧ�����ӷ���
ʽ��
��2������������������Ե�ʵ�飬ѡ����Լ�Ӧ�Ƕ��������
��3����ʹ���Ը��������Һ��ɫ������������Ϊ
��4���������ʼ䷴Ӧ�����ɵ������������ʹ����ʯ��ˮ����ǣ���д���÷�Ӧ�Ļ�ѧ����ʽ��
��5������������(���������е�һ��)�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com