
| A�� | �����£�CH3COONH4��Һ��pH=7���봿ˮ��H2O�ĵ���̶���ͬ | |
| B�� | ��CH3COONH4��Һ����CH3COONa����ʱ��c��NH4+����c��CH3COO-���������� | |
| C�� | �����£���Ũ�ȵ�NH4Cl��CH3COONa����Һ��pH֮��Ϊ14 | |
| D�� | ���µ�Ũ�ȵİ�ˮ�ʹ�������Һ��ˮϡ�͵���ͬ�������ҺpH�ı仯ֵһ����ͬ |
���� A��CH3COONH4��Һ�д�������Ӻ�笠����Ӷ��ܹ�ˮ�⣬pH=7����Ϊ��ˮ��̶���ͬ��
B����CH3COONH4��Һ����CH3COONa����ʱ��c��CH3COO-������c��NH4+����С��
C��NH3•H2O��CH3COOH�ĵ��볣��K��ȣ�������������笠�����ˮ��̶���ͬ��
D���µ�Ũ�ȵİ�ˮ�ʹ�������Һ��ˮϡ����ͬ����ʱ����ҺpH�ı仯ֵһ����ͬ��
��� �⣺A��CH3COONH4��Һ��ˮ�ĵ���̶ȴ��ڴ�ˮ����A����
B����CH3COONH4��Һ����CH3COONa����ʱ����Һ�ʼ��ԣ��ٽ�笠����ӵ�ˮ�⣬c��CH3COO-������c��NH4+����С����B����
C�������������笠�����ˮ��̶���ͬ�������£�����Ũ�ȵ�NH4Cl��CH3COONa����Һ��ϣ���ҺpH=7�����Ե�Ũ�ȵ�NH4Cl��CH3COONa����Һ��pH֮��Ϊ14����C��ȷ��
D����ˮ�ʹ�����Һ�����һ����ͬ�����Ե��µ�Ũ�ȵİ�ˮ�ʹ�������Һ��ˮϡ�͵���ͬ�������ҺpH�ı仯ֵ��һ����ͬ����D����
��ѡC��
���� ���⿼��������������ĵ��볣����ͬʱ�������������������ˮ��̶���ͬ�Լ�Ӱ��ƽ���ƶ������أ���Ŀ�ѶȲ���


 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol•L-1��CH3COOH��Һ�У�c��CH3COO-����c��H+����c��OH-�� | |
| B�� | 0.1mol•L��NaHCO3��Һ�У�c��HCO3-����c��CO32-����c��H2CO3�� | |
| C�� | 0.1mol•L��NH4Cl��Һ�У�c��NH4+��+c��H+��=c��NH3•H2O��+c��OH-�� | |
| D�� | 0.1mol•L�ģ�NH4��2SO4��Һ�У�c��NH4+����c��SO42-����c��H+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | �ѻ� | C�� | �ѽ� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ����a��ʹ���˴����������仯���� | |
| B�� | ��Ӧ����ܼ��ܸ�����������ܼ��� | |
| C�� | ��Ӧ���Ȼ�ѧ����ʽΪ��4HCl��g��+O2��g��$��_{400��}^{����}$2Cl2+2H2O��g��-115.6 kJ | |
| D�� | ����Ӧ����2molҺ̬ˮ���ų�����������115.6kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1L0.1mol•L-1̼������Һ�е�����������Ϊ0.1NA | |
| B�� | ��400mL1mol/L��HNO3��Һ��7gFe�۳�ַ�Ӧ������ת����Ϊ0.375NA | |
| C�� | ��״���£�14g�����й��õ��ӶԵ���ĿΪ1.5NA | |
| D�� | ��֪��ӦN2��g��+3H2��g��?2NH3��g����H=-91.8kJ/mol��ת����Ϊ30%�����ų�91.8kJ����ʱ�����ɰ����ӵ���ĿΪ0.6NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ִ�����Һ�����ʵ���Ũ�ȷֱ�Ϊc1��c2��pH�ֱ�Ϊa�ͣ�a+1��������c1=10c2�� | |
| B�� | 10mL0.1mol•L-1CH3COOH��Һ��������ʵ�����NaOH����Һ����c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | ��0.1mol•L-1NaHSO3��Һ����c��Na+��=c��HSO3-��+c��SO32-��+c��H2SO3�� | |
| D�� | pH��7��ij��Ԫ��������ʽ��NaHA��Һ����c��H+��+2c��A2-��=c��OH-��+c��H2A�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 molCaC2�����к��е���������Ϊ3NA | |
| B�� | ���³�ѹ�£�3g-CH3�к��еĵ�����Ϊ1.8NA | |
| C�� | NA��HCl������22.4LH2��Cl2�Ļ�����������е�ԭ������Ϊ2NA | |
| D�� | 80 mL 12 mol/L��Ũ����������MnO2��Ӧ�����ɵ�Cl2������ĿΪ0.48 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
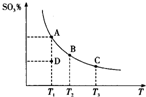 ��������Ĵ�����ԭ��Ϊ2SO2��g��+O2��g��?2SO3��g������Ӧ�����ϵ��ƽ��״̬ʱSO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
��������Ĵ�����ԭ��Ϊ2SO2��g��+O2��g��?2SO3��g������Ӧ�����ϵ��ƽ��״̬ʱSO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��D��ʱ����Ӧ������� | |
| B�� | ��Ӧ2SO2��g��+O2��g��?2SO3��g���ġ�H��0 | |
| C�� | ��B��C���ƽ�ⳣ���ֱ�ΪKB��KC����KB��KC | |
| D�� | ���º�ѹ����ƽ����ϵ��ͨ�뺤����ƽ�������ƶ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com