
��֪:KClO3+6HCl(Ũ) KCl+3Cl2��+3H2O,��ͼ��ʾ,�������Լ��ֱ�����������е���Ӧλ��,ʵ��ʱ��Ũ�������KClO3������,���ñ�����Ǻá��±�����
KCl+3Cl2��+3H2O,��ͼ��ʾ,�������Լ��ֱ�����������е���Ӧλ��,ʵ��ʱ��Ũ�������KClO3������,���ñ�����Ǻá��±����� ʵ������ó��Ľ�����ȫ��ȷ����(��)
ʵ������ó��Ľ�����ȫ��ȷ����(��)
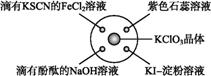
| ѡ�� | ʵ������ | ���� |
| A | ����KSCN��FeCl2��Һ��� | Cl2���л�ԭ�� |
| B | ���з�̪��NaOH��Һ��ɫ | Cl2�������� |
| C | ��ɫʯ����Һ | Cl2����Ư���� |
| D | KI | Cl2���������� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�õ�ʯ�Ʊ�����Ȳ�����г���������H2S���塣����ͼ��������ҩƷ���һ���Ʊ���������Ȳ��װ�ã�����ͨ���ⶨ��Ȳ�������Ӷ������ʯ���ȡ�
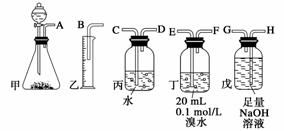
(1)����ʵ��ʱ�����������������������������ȷ����˳����________________(��ӿ���ĸ)��
(2)Ϊ��ʹʵ��������ƽ�ȣ����з�Һ©�����Һ��Xͨ����__________��
(3)���ڱ�״������ˮ����Ȳ��ȫ��Ӧ����C2H2Br4����֪��ȡ��ʯm g�������Ͳ��Һ�����V mL�����ʯ���ȿɱ�ʾΪ_________________________________��
(4)��û�г�H2S��װ�ã��ⶨ�������__________(�ƫ�ߡ�����ƫ�͡����䡱)��������________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڹ�ҵ�ϳɰ��������У��������(����)
A���ڶ������豸����������������£���Ӧ�������ڸ�ѹ�½���
B���¶�Խ��Խ�����ڹ�ҵ�ϳɰ�
C���ڹ�ҵ�ϳɰ���N2��H2��ѭ�����ÿ�����������ʣ����ͳɱ�
D����ʱ�ӷ�Ӧ��ϵ�з��������������ƽ��������Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ���ͬ���ܱ������У��ֱ��������ĵ������������ڲ�ͬ�¶��·�����Ӧ��N2(g)��3H2(g)
 2NH3(g)�����ֱ��ڲ�ͬ��ʱ���ڲⶨ����NH3����������(y������ʾ��)�����ͼ����ͼ��ʾ����ش�
2NH3(g)�����ֱ��ڲ�ͬ��ʱ���ڲⶨ����NH3����������(y������ʾ��)�����ͼ����ͼ��ʾ����ش�
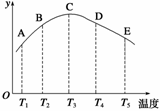
(1)A��B��C��D��E����У��϶�δ��ƽ��ĵ���
________________________________________________________________________��
(2)�˿��淴Ӧ������Ӧ��__________�ȷ�Ӧ��
(3)AC���������������ߣ�CE�����Ǽ��������ߣ��Դӻ�ѧ��Ӧ���ʺͻ�ѧƽ��ĽǶȷ�������˵������
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�й�Al��NaOH��Һ�ķ�Ӧ��˵���У���ȷ���ǣ���
| A�� | ���ǻ�ԭ����������������Al��OH��3 | |
| B�� | NaOH�����������仹ԭ������H2 | |
| C�� | ���ǻ�ԭ����H2O��NaOH���������� | |
| D�� | H2O����������Al������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г�ȥ���ʵķ�������ȷ���ǣ���
| A�� | þ���л����������ۣ���������ռ���Һ��ַ�Ӧ�����ˡ�ϴ�ӡ����� | |
| B�� | �ù�����ˮ��ȥFe3+��Һ�е�����Al3+ | |
| C�� | �����Ƶ���ʯ�ң�ͨ�����������Գ�ȥ�Ҵ��е�����ˮ | |
| D�� | Al��OH��3�л�������Mg��OH��2�����������ռ���Һ����ַ�Ӧ�����ˣ�����Һ��ͨ�����CO2����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������µ�X��Һ���ܸ��������ᷴӦ�����з�Ӧ����������
A.10�� 20 mL 3mol/L��X��Һ B.20�� 30 mL 2mol/L��X��Һ
C.20�� 10 mL 4mol/L��X��Һ D.10�� 10 mL 2mol/L��X��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͬʱ���𱽡���ϩ�ͼ���������ɫҺ�壬�ڿ���ˮԡ���ȵ������£���ѡ�õ��Լ���
A����ˮ B������ C����ˮ��Ũ���� D�����Ը��������Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com