
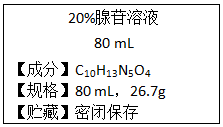
 �������յ������Ϣ���������и��⣺
�������յ������Ϣ���������и��⣺���� ��1��Ħ��������g/molΪ��λ����ֵ������Է���������ȣ����ݦ�=$\frac{���ʵ�����}{��Һ������}$��100%������Һ���������ܼ�������+���ʵ�����=��Һ���������
��2�����ݦ�=$\frac{m}{V}$������Һ���ܶȣ�C=$\frac{1000�Ѧ�}{M}$������Һ�����ʵ���Ũ�ȣ�
��3��������Һϡ�������������ʵ����ʵ������������Ҫ��Һ�������
��� �⣺��1��C10H13N5O4����Է�������Ϊ��12��10+1��13+14��5+16��4=267��Ħ��������g/molΪ��λ����ֵ������Է���������ȣ�������Ħ������Ϊ267g/mol��
���ݦ�=$\frac{���ʵ�����}{��Һ������}$��100%����Һ������Ϊ��$\frac{26.7g}{20%}$=133.5g��133.5g-26.7g=106.8g��
�ʴ�Ϊ��267 g/mol��106.8��
��2������Һ���ܶȦ�=$\frac{133.5g}{80mL}$=1.67g/mL�������Һ�����ʵ���Ũ��C=$\frac{1000��1.67��20%}{267}$=1.25mol/L��
�ʴ�Ϊ��1.25��
��3����ϡ�ͺ���Һ���ΪV��������Һϡ�������������ʵ����ʵ�������ã�80mL��1.25mol/L=0.25mol/L��V�����V=400mL����0.4L��
�ʴ�Ϊ��0.4��
���� ���⿼���������ٷ���Ũ�ȡ����ʵ���Ũ���йؼ��㣬��ȷ�����ʵ���Ϊ���ļ��㹫ʽ����Ϥ��Һϡ�����ǽ���ؼ�����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Һ�м�������ϡ���Ba2++OH-+H++SO42-�TBaSO4+H2O | |
| B�� | NaClO��aq����ͨ����� SO2��C1O-+SO2+H2O=HClO+HSO3- | |
| C�� | ����ͨ��ˮ�У�Cl2+H2O=2H++Cl-+ClO- | |
| D�� | ���������Һ��ͨ������CO2��Ca2++2ClO-+H2O+CO2=CaCO3��+2 HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ���������c��H+������=�ۣ���=�� | |
| B�� | ���ڡ�����Һ��Ϻ�pH=7��������Һ��������ڣ��� | |
| C�� | ������Ģ١��ڡ�����Һ�ֱ����������۷�Ӧ������H2����������� | |
| D�� | ����Һ�м���100mLˮ����Һ��pH���ۣ��ܣ��٣��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 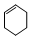 ���ڷ����廯���� ���ڷ����廯���� | B�� |  ���ڱ���ͬϵ�� ���ڱ���ͬϵ�� | ||
| C�� |  ����֬�������� ����֬�������� | D�� | CH3CH��CH3��2������״������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����������������Na2O2���������������ǻ�ԭ�� | |
| B�� | ��Ӧ����������Mn2+ | |
| C�� | ����Ӧ������״����2.24L O2 ʱ����Ӧת�Ƶĵ���Ϊ0.2mol | |
| D�� | ����������Һ�μ�Ũ������Եõ����Ը��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
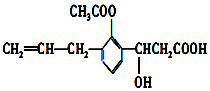 �л���Ľṹ��ʽ��ͼ������л���ɷ����ķ�Ӧ�����У�������
�л���Ľṹ��ʽ��ͼ������л���ɷ����ķ�Ӧ�����У������� | A�� | �٢ڢۢݢ� | B�� | �ڢۢܢݢޢ� | C�� | �ڢۢܢݢޢ� | D�� | �٢ڢۢܢݢޢߢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

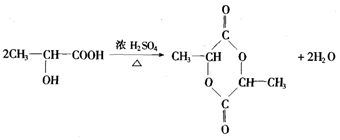
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com