
������������⣺
��1��A��B��C��D��E��F��G�Ļ�ѧʽ��
A____________��B___________��C___________��D___________��E___________��F___________��G___________��
��2��д�������仯�е��ĸ���Ӧ����ʽ
��A��Ũ���Ṳ�ȣ�_________________________________��
��F��G���ã�____________________________________________��
��E��D��Ӧ��____________________________________________��
��A��AgNO3��Һ��ϣ� _________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| c(CuCl 42- ) |
| c[Cu(H2O) 42- ]?c4(Cl-) |
| c(CuCl 42- ) |
| c[Cu(H2O) 42- ]?c4(Cl-) |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ���ƶ���2009��߿�ר�⸴ϰ��ѧ���Ծ����IJ���±�� ���ͣ�022
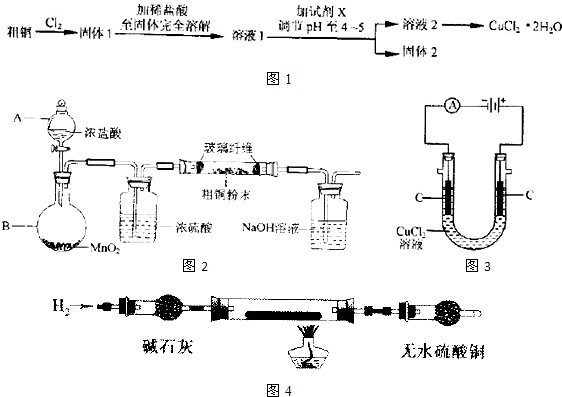
ij��ɫ����A��Ũ�������������²���һ���ڿ�����ð����������F��F���ɫ��ĩG�ڼ���������������һ������E����E��ˮ��Һ����D����Һʱ��������������A��B������B��ʹ������Һ�����������ɫ����A����Һ�е���AgNO3��Һ������������ɫ������C���ð�ɫ����������ϡHNO3�У���D�ν�����ɫʵ�飬����ɫ�ܲ����۲쵽����ʱ�������ɫ��
�Իش�
(1)A��B��C��D��E��F��G�Ļ�ѧʽ��
A________��B________��C________��
D________��E________��F________��
G________��
(2)д�������仯�е��ĸ���Ӧ����ʽ
��A��Ũ���Ṳ�ȣ�________��
��F��G���ã�________��
��E��D��Ӧ��________��
��A��AgNO3��Һ��ϣ�________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ����A��Ũ�������������²���һ���ڿ�����ð����������F��F���ɫ��ĩG�ڼ���������������һ������E����E��ˮ��Һ����D����Һʱ��������������A��B������B��ʹ������Һ�����������ɫ����A����Һ�е���AgNO3��Һ������������ɫ������C���ð�ɫ����������ϡHNO3�У���D�ν�����ɫʵ�飬����ɫ�ܲ����۲쵽����ʱ�������ɫ��
�Իش𣺣�1��A��B��C��D��E��F��G�Ļ�ѧʽ��
A___________��B___________��C___________��
D___________��E___________��F___________��G___________��
��2��д�������仯�е��ĸ���Ӧ����ʽ
��A��Ũ���Ṳ�ȣ�_________________________________��
��F��G���ã�_____________________________________��
��E��D��Ӧ��_____________________________________��
��A��AgNO3��Һ��ϣ�_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ����A��Ũ�������������²���һ���ڿ�����ð����������F��F��Ũ��Һ���ɫ��ĩG�ڼ���������������һ������E����E��ˮ��Һ����D����Һʱ��������������A��B������B��ʹ������Һ�����������ɫ����A����Һ�е���AgNO3��Һ������������ɫ������C���ð�ɫ����������ϡHNO3����D�ν�����ɫʵ�飬����ɫ�ܲ����۲쵽����ʱ�������ɫ��
������������⣺
��1��A��B��C��D��E��F��G�Ļ�ѧʽ��
A____________��B___________��C___________��D___________��E___________��F___________��G___________��
��2��д�������仯�е��ĸ���Ӧ����ʽ
��A��Ũ���Ṳ�ȣ�_________________________________��
��F��G���ã�____________________________________________��
��E��D��Ӧ��____________________________________________��
��A��AgNO3��Һ��ϣ� _________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com