
��ѧϰС��ͨ���������Ϸ�����KClO3��Ũ����Ҳ�����Ʊ�������Ϊ�˸�С��������ͼװ����ʵ������ȡCl2��̽�����й����ʣ�
��1��װ��A�д���һ������������� ��
��2��д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3��Ϊ�˳�ȥ�����е��Ȼ��⣬��Ҫ��.A��B֮��װ��������װ��E��װ��E����ʢװ���Լ�Ϊ ��Ϊ��̽��ʹ��ɫ������ɫ������������������ˮ��Ӧ���������Ҫ������װ��������װ��E�����м���Ũ���ᣬװ��E������װ���е�����λ��Ϊ ��ѡ����ţ���
a A��B֮�� b��B��C֮��
c��C��D֮�� d��D֮��
��4��ȡ�¼���ƿB������˿����������״̬�����뵽����ƿB�У����Թ۲쵽�������� ���ܼ���÷�Ӧ��������Ԫ�صļ�̬���Լ� ��ѡ����ţ���ѡ����
a����ˮ b������ˮ������
c������ˮ������������Һ d������ˮ��KSCN��Һ
��5������һ����ѧѧϰС��ͻ�����������ָ��������װ�õ�ȱ�ݣ��������˸Ľ���ʩ���øĽ���ʩ�ǣ� ��
��1���ij���©��Ϊ��Һ©�� ��1�֣�
��2��KClO3 +6HCl =" KCl" +3Cl2��+ 3H2O ��3�֣�
��3������ʳ��ˮ��1�֣���b ��2�֣�
��4������ƿ�ڼ�ƿ�����ػ�ɫ�̲��� ��2�֣���bcd ��3�֣�
��5����װ��D������һ��ʢװŨ��Һ��װ�ã�ʹ��Һ�����ݳ�������β�� ��3�֣�
�������������ʵ��Ŀ�ģ�������ͼװ����ʵ������ȡCl2��̽�����й����ʣ���װ��A��ȡ������KClO3 +6HCl
=" KCl" +3Cl2��+ 3H2O ����װ��C��D֤�����������������Ư���ԣ���ʪ����������Ư���ԣ���Ҫ��
��ͨ��װ��C����������Ϊ��������������װ��Cǰ������ʢ��Ũ�����ϴ��ƿ����1��װ��A�иij�
��©��Ϊ��Һ©������Һ©�����ڿ��Ʒ�Ӧ�Ľ��С���2��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��KClO3 +6HCl
=" KCl" +3Cl2��+ 3H2O ����3��װ��C��D֤�����������������Ư���ԣ���ʪ����������Ư���ԣ���Ҫ
����ͨ��װ��C����������Ϊ��������������װ��Cǰ������ʢ��Ũ�����ϴ��ƿ����4����˿������
�о���ȼ�գ������ػ�ɫ�̣�����ˮ�õ���ɫ��Һ����5����װ��D������һ��ʢװŨ��Һ��װ�ã�ʹ
��Һ�����ݳ�������β������ֹ��Ⱦ������
���㣺�����������Ʊ���������ѧ���ʵļ��顣


 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣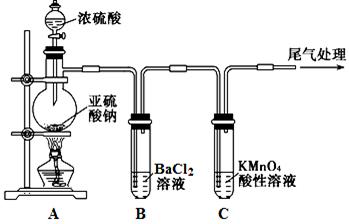
��֪��Na2SO3��H2SO4(Ũ)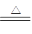 Na2SO4��SO2����H2O
Na2SO4��SO2����H2O
��ش��������⣺
��1��װ��A��ʢ��Ũ��������������� ��
��2��ʵ������У�C�е������� ��˵��SO2���� �ԡ�
��3��ʵ������У��۲쵽װ��B�г��������Եİ�ɫ������Ϊ̽���ð�ɫ�����ijɷ֣���С��ͬѧ����������ʵ�飺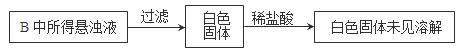
����ʵ����ʵ�жϸð�ɫ�����ijɷ��� ���ѧʽ���������ð�ɫ������ԭ������� ������ĸ��ţ���
a��BaSO3�Ȳ�����ˮҲ��������
b��BaCl2��Һ�п����ܽ�������
c��BaCl2��Һ�п��ܻ���NaOH
d����A�Ƶõ�SO2�����п��ܻ�������
��4�������װ��A�е�ŨH2SO4����ŨHNO3���Դ�ʵ���Ƿ���Ӱ�첢������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ̽���������Ƶ����ȶ��ԣ�ij�о���ѧϰС�齫��ˮ�������Ƹ���������
�ȣ����������Ⱥ�Ĺ�����������ͼ��ʾ��ʵ��װ�ý���ʵ�顣��ش������й����⣺
��1���������ϣ���ˮ�������Ƹ����������ȵ�600��ſ�ʼ�ֽ⣬�ҷֽ����ֻ������
������һ�ֹ��塣��������¶ȵ���600�棬��������ȴ����������л����μ�ϡ������
�������ڵμ�ϡ��������������� �����ʵ���Ũ�ȱ仯����Ϊ ��
�����ʵ���Ũ�ȱ仯����Ϊ ��
��2����������¶�Ϊ700�棬��������ȴ����������л����μ�ϡ�������������۲쵽��ƿ�г��ֵ���ɫ���������д������ݲ�������Ӧ���ɵ���ɫ���������ӷ���ʽΪ ����ʱ��B��C��װ���п��ܹ۲쵽������Ϊ ��
��3���ڣ�2���еμ������������ƿ�ڳ� �⣬��������һ��Ũ�Ƚϴ�������ӡ�Ϊ����������ӣ���ȡ������������ˮ�����Һ�������Ǽ���������ӵ�����ʵ�鷽������Ϊ�����ķ����� ����ס����ҡ�������˵����һ������������ԭ�� ��
�⣬��������һ��Ũ�Ƚϴ�������ӡ�Ϊ����������ӣ���ȡ������������ˮ�����Һ�������Ǽ���������ӵ�����ʵ�鷽������Ϊ�����ķ����� ����ס����ҡ�������˵����һ������������ԭ�� ��
�����ף�ȡ����������Һ���Թ��У��ȼ�ϡ ���ټ�
���ټ� ��Һ���а�ɫ�������ɣ�֤�������Ӵ��ڡ�
��Һ���а�ɫ�������ɣ�֤�������Ӵ��ڡ�
�����ң�ȡ����������Һ���Թ��У��ȼ�ϡHCl���ټ� ��Һ���а�ɫ�������ɣ�֤�������Ӵ��ڡ�
��Һ���а�ɫ�������ɣ�֤�������Ӵ��ڡ�
��4��д�� ������ȵ�600�����Ϸֽ�Ļ�ѧ����ʽ ��
������ȵ�600�����Ϸֽ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧ��ȤС����̽������Ũ����ķ�Ӧʱ�����̲���ͭ��Ũ���ᷴӦ��ʵ��װ��(ͼ��)��Ϊ��ͼ����ʾ��װ�á�

��ش��������⣺
��1��д��ͼ�����������Ӧ�Ļ�ѧ����ʽ_________��
��2��ͼ��ʵ��װ����ͼ����Ƚϣ����ŵ��ǣ����ܸ��õ������ж�����SO2����ֹ����Ⱦ��������_________��
��3�����жԵ���a�����÷�������ȷ����_________ (����ĸ)��
A�����ȷ�Ӧ�����У��������ƶ�����a�����������
B��ֹͣ���ȣ��Թ��ڵ�ѹǿ��С���ӵ���a����Ŀ����������Թ�A�ڵ�ѹǿ����ֹ����
C��ֹͣ��Ӧ����װ��֮ǰ������a��ͨ����������ų�װ���ڵ�SO2���壬��ֹ����Ⱦ����
��4����Ӧһ��ʱ����Թ�Aȡ�£�Ȼ���Թ�A����Һ������һʢ������ˮ���Թ�D�С���С��ͬѧΪȷ����Һ�������ڵĽ������ӣ���������̽�����̡�
��������裺
�����ֻ����Fe3���������_________�������_________��
��ʵ����ƣ�
�ֱ�ȡ�����Թ�D����Һ��ѡ���ṩ���Լ�����Ƽ�ʵ�������Һ�������ڵĽ������ӡ�����д�±��հ�(���Բ�������Ҳ��������)��
�ṩ���Լ���ϡ���ᡢϡ���ᡢKSCN��Һ��KMnO4��Һ��
| ���� | ��ѡ�Լ� | ������ |
| 1 | | |
| 2 | | |
| 3 | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧʵ��С��Ϊ����֤SO2��Cl2��Ư���ԣ����������ͼ��ʾ��ʵ��װ�á���ش��������⣺
��1�������Ʊ�SO2��Cl2�����ݵ�ԭ���ֱ��ǣ�Na2SO3+H2SO4=Na2SO4+H2O+SO2����MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O������ͼA��Eװ����������Cl2��װ����
MnCl2+Cl2��+2H2O������ͼA��Eװ����������Cl2��װ����
������ţ�����Ӧ�����������ֵ������� ��
��2����Ӧ��ʼ����B��D�Թ��е�Ʒ����Һ����ɫ��ֹͣͨ����B��D�����Թ��е���Һ���ȣ�B�Թ��е������� ��
��3��װ��C�������� ��
��4��NaOH����������Һ�ֱ����������巴Ӧ�����ӷ���ʽ�� �� ��
��5����С��ͬѧ�����������Ϻ�ͨ��Ʒ����Һ��һ��ʱ���Ʒ����Һ��������ɫ���������ϵ�֪���������尴�����1��1��ϣ�����ˮ��Ӧ���������ֳ����ᣬ���ʧȥƯ�����á��÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ����С��ͨ��ʵ���о�NO2�����ʡ�
��֪��2NO2��2NaOH��NaNO3��NaNO2��H2O
������ͼ1��ʾװ��̽��NO2�ܷ�NH3��ԭ��K1,K2Ϊֹˮ�У��г̶ֹ�װ����ȥ��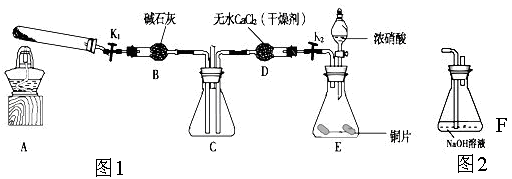
(1)Eװ������ȡNO2��Ӧ�����ӷ���ʽ�� ��
(2)��ʵ������ȡ����ʱ��ֻ��һ���Լ���������������ѡȡ ��
a��NH4HCO3 b��NH4Cl c��Ũ��ˮ
(3)��NO2�ܹ���NH3��ԭ��Ԥ�ڹ۲쵽Cװ���е������� ��
(4)ʵ����������������ã���δ�ܹ۲쵽Cװ���е�Ԥ������С��ͬѧ������ԭ������ǣ�
�٣���ԭ�Խ��������ܽ�NO2��ԭ�����ڴ������£�NO2��ת���ʼ��ͣ�
�� ��
(5)��ʵ��װ�ô���һ�����Ե�ȱ���� ��
(6)Ϊ����֤NO2�ܱ�Na2O2��������С��ͬѧѡ��B��D��Eװ�ã���B�е�ҩƷ����ΪNa2O2����ѡFװ�ã���ͼ2��ʾ����������װ������ʵ�顣װ�õĺ�������˳����____��ʵ������У�Bװ���е���ɫ��ĩ��ɰ�ɫ�������飬�ð�ɫ����Ϊ��������������������ɡ��Ʋ�Bװ���з�Ӧ�Ļ�ѧ����ʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪A��B��C��D֮���ת����ϵ��ͼ��ʾ������˵����ȷ����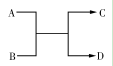
A����AΪFe��DΪ��������Bһ��Ϊ��
B����A��DΪ�����BΪˮ����Cһ�������嵥��
C����A��B��C��D��Ϊ������÷�Ӧһ�����ڸ��ֽⷴӦ
D����A��B��C��D��Ϊ10����������C�ǿ�ʹʪ��ĺ�ɫʯ����ֽ���������壬��D�ڳ�����һ����Һ̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ס��ҡ���������Ϊ��ѧ��ѧ���������ʣ�����֮���ת����ϵ����ͼ(��Ӧ������������������ȥ)��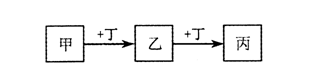
����˵������ȷ����
| A��������AlCl3��Һ��������NaOH��Һ |
| B��������Fe��������Cl2 |
| C��������CO2�������ΪMg |
| D��������O2�����ҡ�������Է��������������16 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ס��ҡ����������������У��ס��ҡ�����������ͬ��ij��Ԫ�أ�����֮���������ת����ϵ��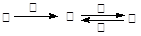 �������й����ʵ��ƶϲ���ȷ����(�� ��)
�������й����ʵ��ƶϲ���ȷ����(�� ��)
| A������Ϊ��̿��������O2 | B������ΪSO2�������ǰ�ˮ |
| C������ΪFe������������ | D������ΪNaOH ��Һ��������AlCl3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com