
C(s)+H2O(g)![]() CO(g)+H2(g)��DH��0
CO(g)+H2(g)��DH��0
��֪ƽ��ʱ��COΪ0.12mol������գ�
(1)����v(H2O)��ʾ�÷�Ӧǰ20s��ƽ�����ʣ���v(H2O)=________��
(2)��������ƽ�������м�������Na2O���壬���ڴ��¶����ٴδﵽ�µ�ƽ��(�ڶ�ƽ��)����ƽ��ʱH2�����ʵ���________(�������С�����䡱)��������________��
(3)����������һƽ��Ļ�������ٳ���amolH2(a��0.12)����ͬ�����´ﵽ�µ�ƽ��(����ƽ��)����ʱCO�����ʵ���n�ķ�Χ��________��
| (1)0.0030mol��L-1��s-1
(2)��С,Na2O��H2O��Ӧ��ʹˮ����Ũ�Ƚ��ͣ�ƽ�������ƶ� (3)(0.12-a)��n��0.12 ������(1)20s����CO��ʾ�÷�Ӧ��ƽ������v(CO)=(0.12mol��2L)/20 s=0.003mol��L-1��s-1�� ���ݣ����ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ���H2O��ʾ�÷�Ӧǰ20s�ڵ�ƽ������v(H2O)=0.003mol��L-1��s-1�� (2)����Na2O��H2O��Ӧ��ʹˮ����Ũ�Ƚ��ͣ�ƽ�������ƶ�����˵ڶ���ƽ��ʱ��H2��Ũ�ȼ�С�� (3)��һ��ƽ��ʱCO��H2���ʵ�����Ϊ0.12mol������amolH2��ƽ�������ƶ���CO�����ʵ�����С���ﵽƽ��(����ƽ��)ʱ��CO�����ʵ���С��0.12mol��������������ԭ��������amolH2��ѧƽ����������ָı�ķ����ƶ�(���ܵ������ָı�)����˱仯��H2�����ʵ���С��amol������ƽ��ʱCO�����ʵ�������(0.12-a)mol��
|


 ��ҵ����ϵ�д�
��ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
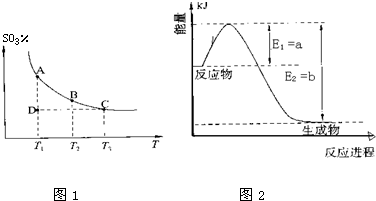
| ||
| ���� |
| ||
| ���� |
| 4 |
| 7 |
| ||
| ���� |
| T/�� | 200 | 300 | 400 |
| K | K1 | K2 | 0.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����֪��N2(g)+O2(g)=2NO(g)����H= +180.5kJ/mol
4NH3(g)+5O2(g)=4NO(g)+6H2O(g)����H=��905kJ/mol
2H2(g)+O2(g)=2H2O(g)����H=��483.6kJ/mol
��N2(g)+3H2(g)=2NH3(g)�ġ�H= ��
��2����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g) ![]() 2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
������ѹǿ ��ͨ��He
��ʹ�ô��� �ܽ����¶�
��3����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g) ![]() 2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ![]() ������������´ﵽƽ��ʱN2ת����Ϊ ��
������������´ﵽƽ��ʱN2ת����Ϊ ��
��4���ϳɰ���ԭ��������һ�����͵���ɫ��Դ�����й����ķ�չǰ������������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飺������c��d��Ϊ̼����NaCl��Һ�����Ϊ500ml��

��b��Ϊ �����缫��Ӧʽ ��
c��Ϊ �����缫��Ӧʽ
����ͼװ���У���b�������ĵ�O2�ڱ�״���µ����Ϊ280mlʱ�����ҳ���Һ��PHΪ �����跴Ӧǰ����Һ������䣬��NaCl��Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����֪��N2(g)+O2(g)=2NO(g)����H= +180.5kJ/mol
4NH3(g)+5O2(g)=4NO(g)+6H2O(g)����H=��905kJ/mol
2H2(g)+O2(g)=2H2O(g)����H=��483.6kJ/mol
��N2(g)+3H2(g)=2NH3(g)�ġ�H= ��
��2����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g) 2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
������ѹǿ ��ͨ��He
��ʹ�ô��� �ܽ����¶�
��3����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g) 2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
![]() ������������´ﵽƽ��ʱN2ת����Ϊ ��
������������´ﵽƽ��ʱN2ת����Ϊ ��
��4���ϳɰ���ԭ��������һ�����͵���ɫ��Դ�����й����ķ�չǰ������������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飺������c��d��Ϊ̼����NaCl��Һ�����Ϊ500ml��
��b��Ϊ �����缫��Ӧʽ ��
c��Ϊ �����缫��Ӧʽ
����ͼװ���У���b�������ĵ�O2�ڱ�״���µ����Ϊ280mlʱ�����ҳ���Һ��PHΪ �����跴Ӧǰ����Һ������䣬��NaCl��Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011������ʡ���Ը���ѧ����1���¿������ۣ���ѧ���� ���ͣ������
��1����֪��N2(g)+O2(g)=2NO(g)����H= +180.5kJ/mol
4NH3(g)+5O2(g)=4NO(g)+6H2O(g)����H=��905kJ/mol
2H2(g)+O2(g)=2H2O(g)����H=��483.6kJ/mol
��N2(g)+3H2(g)=2NH3(g)�ġ�H= ��
��2����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g)  2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
������ѹǿ ��ͨ��He
��ʹ�ô��� �ܽ����¶�
��3����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g)  2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ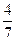 ������������´ﵽƽ��ʱN2ת����Ϊ ��
������������´ﵽƽ��ʱN2ת����Ϊ ��
��4���ϳɰ���ԭ��������һ�����͵���ɫ��Դ�����й����ķ�չǰ������������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飺������c��d��Ϊ̼����NaCl��Һ�����Ϊ500ml��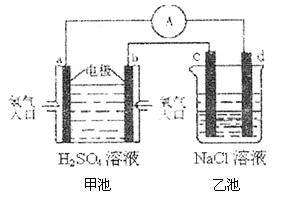
��b��Ϊ �����缫��Ӧʽ ��
c��Ϊ �����缫��Ӧʽ 
����ͼװ���У���b�������ĵ�O2�ڱ�״���µ����Ϊ280mlʱ�����ҳ���Һ��PHΪ �����跴Ӧǰ����Һ������䣬��NaCl��Һ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com