
£Ø13·Ö£©¢ÅĶź³ÉĻĀĮŠĪļÖŹµÄµē×ÓŹ½£ŗ
KOH £» H2S £»
CS2 £» NH4Cl £»
¢ĘĻĀĮŠĪ¢Į£Ö®¼ä£¬ŹōÓŚĶ¬Ī»ĖŲµÄŹĒ £¬£ØĢīŠņŗÅ£¬ĻĀĶ¬£©ŹōÓŚĶ¬ĖŲŅģŠĪĢåµÄŹĒ £¬ŹōÓŚĶ¬·ÖŅģ¹¹ĢåµÄŹĒ £¬¹ĢĢåŹ±ŹōÓŚŌ×Ó¾§ĢåµÄŹĒ ”£
A£®Õż¶”ĶéÓėŅģ¶”Ķé B£®ŗģĮ×Óė°×Į× C£®ėÓėė°
D£®ĀČ»ÆÄĘÓėøɱł E£®½šøÕŹÆÓėĖ®¾§ F£®ŃõĘųŗĶ³ōŃõ
G£® 13CŗĶ14C H£®CH3OCH3ŗĶCH3CH2OH
¢ĒŃĒĮņĖįÄĘŗĶµāĖį¼ŲŌŚĖįŠŌČÜŅŗÖŠ·¢ÉśŅŌĻĀ·“Ó¦£ŗ
Na2SO3+ KIO3+ H2SO4”Ŗ”Ŗ Na2SO4+ K2SO4+ I2+ H2O
£Øa£©ÅäĘ½ÉĻĆęµÄŃõ»Æ»¹Ō·“Ó¦·½³ĢŹ½£¬½«ĻµŹżĢīČė·½æņÖŠ”£
£Øb£©ĘäÖŠŃõ»Æ¼ĮŹĒ £¬Čō·“Ó¦ÖŠÓŠ5 molµē×Ó×ŖŅĘ£¬ŌņÉś³ÉµÄµāŹĒ mol”£
£Ø13·Ö£©£Ø1£©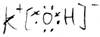
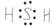
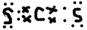
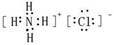 £»
£»
¢Ę CG £¬ BF £¬ AH £¬ E ”£
¢Ē£Øa£©5 Na2SO3+ 2 KIO3+ 1 H2SO4£½ 5 Na2SO4+ 1 K2SO4+ 1 I2+ 1 H2O £Øb£© KIO3 £¬ 0.5
”¾½āĪö”æ£Ø2£©æ¼²éĮĖø÷øÅÄīÖ®¼äµÄ²ī±š”£ÖŠ×ÓŹż²»Ķ¬”¢ÖŹ×ÓŹżĻąĶ¬µÄŌ×Ó»„³ĘĪŖĶ¬Ī»ĖŲ£»Ķ¬ÖÖŌŖĖŲ²»Ķ¬µ„ÖŹ»„³ĘĪŖĶ¬ĖŲŅģŠĪĢ壬·Ö×ÓŹ½ĻąĶ¬µÄ²»Ķ¬ĪļÖŹ»„³ĘĪŖĶ¬·ÖŅģ¹¹Ģ壻


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
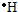
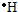
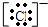
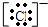








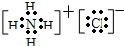
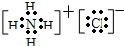
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ½ĖÕŹ”ŅĒÕ÷֊ѧ2011£2012ѧğøßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗ022
(1)Ķź³ÉĻĀĮŠĪļÖŹµÄµē×ÓŹ½£ŗKOH________£»H2S________£»CS2________£»NH4Cl________£»
(2)ĻĀĮŠĪ¢Į£Ö®¼ä£¬ŹōÓŚĶ¬Ī»ĖŲµÄŹĒ________£¬(ĢīŠņŗÅ£¬ĻĀĶ¬)ŹōÓŚĶ¬ĖŲŅģŠĪĢåµÄŹĒ________£¬ŹōÓŚĶ¬·ÖŅģ¹¹ĢåµÄŹĒ________£¬¹ĢĢåŹ±ŹōÓŚŌ×Ó¾§ĢåµÄŹĒ________£®
A£®Õż¶”ĶéÓėŅģ¶”Ķé
B£®ŗģĮ×Óė°×Į×
C£®ėÓėė°
D£®ĀČ»ÆÄĘÓėøɱł
E£®½šøÕŹÆÓėĖ®¾§
F£®ŃõĘųŗĶ³ōŃõ
G£®13CŗĶ14C
H£®CH3OCH3ŗĶCH3CH2OH
(3)ŃĒĮņĖįÄĘŗĶµāĖį¼ŲŌŚĖįŠŌČÜŅŗÖŠ·¢ÉśŅŌĻĀ·“Ó¦£ŗ
________Na2SO3£«________KIO3£«________H2SO4”Ŗ”Ŗ________Na2SO4£«________K2SO4£«________I2£«________H2O
(a)ÅäĘ½ÉĻĆęµÄŃõ»Æ»¹Ō·“Ó¦·½³ĢŹ½£¬½«ĻµŹżĢīČė·½æņÖŠ£®
(b)ĘäÖŠŃõ»Æ¼ĮŹĒ________£¬Čō·“Ó¦ÖŠÓŠ5 molµē×Ó×ŖŅĘ£¬ŌņÉś³ÉµÄµāŹĒ________mol£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ½ĖÕŹ”ŅĒÕ÷֊ѧøßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø13·Ö£©¢ÅĶź³ÉĻĀĮŠĪļÖŹµÄµē×ÓŹ½£ŗ
KOH £» H2S £»
CS2 £» NH4Cl £»
¢ĘĻĀĮŠĪ¢Į£Ö®¼ä£¬ŹōÓŚĶ¬Ī»ĖŲµÄŹĒ £¬£ØĢīŠņŗÅ£¬ĻĀĶ¬£©ŹōÓŚĶ¬ĖŲŅģŠĪĢåµÄŹĒ £¬ŹōÓŚĶ¬·ÖŅģ¹¹ĢåµÄŹĒ £¬¹ĢĢåŹ±ŹōÓŚŌ×Ó¾§ĢåµÄŹĒ ”£
| A£®Õż¶”ĶéÓėŅģ¶”Ķé |
| B£®ŗģĮ×Óė°×Į× |
| C£®ėÓėė° |
| D£®ĀČ»ÆÄĘÓėøɱł |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ½ĖÕ×ØĻīĢā ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com