
�ҹ����ϡ������ȵ�ʢ����ɽ������������ȩ�����ܸߣ����������ɴﵽ60%��90%������ȩҲ�������������ϩΪԭ���˹��ϳɣ�����ȩ�ֿ������ϳ�������ͪ���㾫���ϣ���ϳ�·�����£�

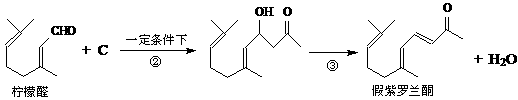
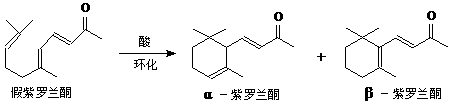
��֪����
��
��ͬһ̼ԭ����������˫���ṹ���ȶ���
�Ը�������ת����ϵ�ش��������⣺
��1��д��A�Ľṹ��ʽ ��C�Ľṹ��ʽ ��
��2���ٶ�Ӧ�ķ�Ӧ������ ����Ӧ�۵ķ�Ӧ������ ��
��3��д��Bת��Ϊ����ȩ�Ļ�ѧ����ʽ ��
��4�����ݷ�Ӧ�ڵķ�Ӧ����д��CH3CHO��������HCHO��Ӧ����Ľṹ��ʽ��
��
��5����������ȩ�к���̼̼˫����ʵ�鷽���ǣ� ��
��6������������ͪ���£�������ͪ�кܶ�ͬ���칹�壬����������������ͬ���칹���� �֡�
�ٺ���һ������ �����ڴ����Ҳ��ܷ�����������Ӧ
�ۺ˴Ź���������ʾ��5����
��14�֣���1��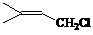 ��2�֣�
��2�֣�  ��2�֣�
��2�֣�
��2��NaOH��ˮ��Һ������ ��2�֣� ��ȥ��Ӧ ��1�֣�
��3��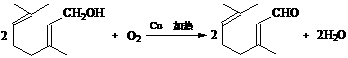 ��2�֣�
��2�֣�
��4��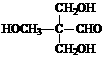 ��2�֣�
��2�֣� 
��5����ʢ���������CCl4��Һ���Թ��еμ�����ȩ���ߵα�������Һ��Ϊ��ɫ���֤������ȡ��������ȩ������������Һ��ϲ�������ˮԡ�м��ȣ���ַ�Ӧ��ȡ������Һ������һ�Թ��У����μӸ������������Һ������������������Һ��ɫ����֤������ȩ���ӽṹ����̼̼˫������ ��2�֣�
��6��2 ��1�֣�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ǹ��ʹ��ϵĸ�Ч��ȫɱ������������ҵ�Ʊ�ClO2�ķ�Ӧԭ�������ã�2NaClO3+4HCl═2ClO2��+Cl2��+2H2O+2NaCl�����й��ڸ÷�Ӧ��˵������ȷ���ǣ�������
| �� | A�� | Ũ�����ڷ�Ӧ�н����ֻ�ԭ�� |
| �� | B�� | ÿ����0.1mol ClO2ת��0.5mol���� |
| �� | C�� | �����ԣ�NaClO3��ClO2 |
| �� | D�� | �������ͱ���ԭ�����ʵ����ʵ���֮��Ϊ1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҩ�ǰѲݵijɷ�֮һM��������ɱ������,M�Ľṹ��ͼ��ʾ������������ȷ����( )

A.M����Է���������180
B.1 mol M�������2 mol Br2������Ӧ
C.M��������NaOH��Һ������Ӧʱ�������л�����Ļ�ѧʽΪC9H4O5Na4
D.1 mol M������NaHCO3��Ӧ������2 mol CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����KCl��Һ��K2CO3���Լ���________�����ӷ���ʽΪ
________________________________________________________________________��
(2)��ȥFeCl2��Һ�л��е�FeCl3��________�����ӷ���ʽΪ
________________________________________________________________________��
(3)��ֽ�����˶�����������ϴʱ������ʹ�á�����顱(��Ҫ�ɷ�������)�롰84����Һ��(��Ҫ�ɷ���NaClO)���������ж����¼����Ը�����Ļ�ѧ֪ʶ������ԭ����(�����ӷ���ʽ��ʾ)________________________________________________________________
________________________________________________________________________��
(4)��A��B��C��D������ɫ���壬��A��ʹʪ��ĺ�ɫʯ����ֽ��������һ�������£�A������B��Ӧ����C��C��������Ϊ����ɫ����D��A������ɰ��̣���D��Ũ��Һ�����̿�(��Ҫ�ɷ���MnO2)�ڼ��������·�Ӧ����ȡ����ɫ����E����Ҫ��ش��������⣺
a��д��ʵ������A����Ļ�ѧ��Ӧ����ʽ
________________________________________________________________________��
b��д���ڻ�ѧ��Ӧ����ʽ
_________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Դ���������й㷺��;��������Ni���������缫���ŨNaOH��Һ�Ʊ���������Na2FeO4��װ����ͼ��ʾ�������ƶϺ�������

A�������������缫��ӦΪFe��6e-+4H2O=FeO42-+ 8H+
B�����ʱ���ӵ���������Ϊ��������Ni�缫����Һ��Fe�缫������
C������ĤΪ�����ӽ���Ĥ����OH-���������ƶ�
D�����ʱ������pH���͡�������pH���ߣ���ȥ��Ĥ��Ϻ���ԭ��Һ�Ƚ�pH���ͣ�������ǰ������仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1 mol ij��̬��CxHy��ȫȼ��,����3 mol O2,��( )
A.x=2,y=2 B.x=2,y=4
C.x=3,y=6 D.x=3,y=8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���A����Ϊֲ���������ڼ���Ϊ����ʹ�ã�ͨ�������Ƴɻ�����
 D( )��D�������������»Ỻ���ͷų�A���ϳ�D��һ��
D( )��D�������������»Ỻ���ͷų�A���ϳ�D��һ��
�����������ʼ�ת����ϵ��ͼ��ʾ��
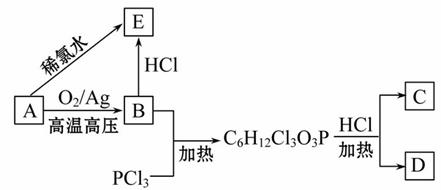
��ش��������⣺
(1)A��������_________��A��������Ӧ������C��C��������_________��
(2)���ⶨE�к�����Ԫ�أ���E���������ᷢ��������Ӧ����E�Ľṹ��ʽΪ_________����Aֱ������E�ķ�Ӧ������_________��
(3)�������������£�D��ˮ��Ӧ����A�Ļ�ѧ����ʽΪ______________________��
(4)д��E������ͬ���칹��Ľṹ��ʽ__________________��
A����Ϊֲ���������ڼ�����AΪ��ϩ��
�ɵ�(2)�ʡ�E�к�����Ԫ�أ���E���������ᷢ��������Ӧ��֪E�к�����Ԫ�غ��ǻ������E��������ϩ��ϡ��ˮ��Ӧ�õ���֪EΪClCH2CH2OH��������Ϊ��CH2��CH2��HO��Cl�ӳɵIJ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.6 mol X�����0.4 mol Y���������ݻ�Ϊ2 L��������,ʹ�䷢�����·�Ӧ:3X(g)+Y(g) nZ(g)+2W(g)��5 minĩ����0.2 mol W,����֪��ZŨ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ0.01 mol��L-1��min-1,��n��ֵ
nZ(g)+2W(g)��5 minĩ����0.2 mol W,����֪��ZŨ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ0.01 mol��L-1��min-1,��n��ֵ
��(����)
A.4�������� B.3�������� C.2�������� D.1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ȳ��һ����Ҫ���л�����ԭ�ϣ�����ȲΪԭ���ڲ�ͬ�ķ�Ӧ�����¿���ת�������»����

�ش��������⣺
(1)����������̼̼������Ϊ_________�������ȡ��������_________�֡�
(2)������ϩ����Ȳ���ӵ�˵���������_________(����ĸ����)��
A.��ʹ����KMnO4��Һ��ɫ
B.1 mol��ϩ����Ȳ�������3 mol Br2�����ӳɷ�Ӧ
C.��ϩ����Ȳ�����ں������ֹ�����
D.����������Ȳ����ϩ����Ȳ��ȫȼ��ʱ�ĺ���������ͬ
E.��ϩ����Ȳ�����е�����ԭ��һ������ƽ��
(3)������ϩ�ķ���ʽΪ________________��д���뻷����ϩ��Ϊͬ���칹�������ڷ������ķ��ӵĽṹ��ʽ��____________________��
(4)�������ڱ���ͬϵ�����_________(����ĸ����)��
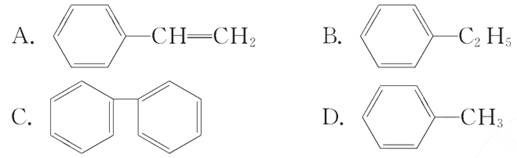
(5)��ֱ�д����Ȳ��CH3CHO��CH3COOH��CH3COOC2H5���������ŵ�����___________��
___________��___________��___________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com