
��16�֣���1�����к͵ζ����������У������¸�����������������ʵ�����á�ƫ�ߡ�����ƫ�͡�����Ӱ�족��գ�
�ٵζ���������ˮϴ����δ����֪Ũ�ȵı���Һ��ϴ��ʹ�ζ���� ��
����ƿ������ˮϴ�������ô�����Һ��ϴ��ʹ�ζ���� ��
�۵ζ���(װ����Һ)�ڵζ�ǰ���촦�����ݣ��ζ����������ݣ�ʹ�ζ���� ��
�ܵζ�ǰƽ�ӣ��ζ����˸��ӣ�ʹ�ζ���� ��
���ú�Na2O���ʵ�NaOH������������֪Ũ�ȵı���Һ�����ڵζ�δ֪Ũ�ȵ����ᣬʹ��������Ũ�� ��
��ϴ����ƿʱ�����ϡʳ��ˮ��������ˮ��Ȼ������ƿװ��������ᣬ��NaOH����Һ�ζ�ʱ���Բ�õĽ�� ��
��2����֪H+(aq)+OH-(aq) = H2O(l) ��H= ��57.3kJ��mol��1����50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
�ٴ�ʵ��װ���Ͽ���ͼ����ȱ��һ�ֲ��������� ��
�ڴ��ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ����ƫС������Ӱ�족)��
����ͨ��ʵ��ⶨ�к��ȵĦ�H�������������ڣ�57.3kJ��mol��1����ԭ������ǣ�
��
��3���ֱ���ƻ�ѧʵ�飬����ѷ���֤����������ˮʱ���������б仯(��ѡ�õ�ҩƷ��������������Һ��ʯ����Һ����̪��Һ��pH��ֽ���ƾ���)��
��֤������������ˮ�ⷴӦ ��
��֤����ˮ�ⷴӦ��һ�����ȷ�Ӧ ��
��1����ƫ�� ����ƫ�� ����ƫ�ߡ� ��ƫ�͡�����ƫ�͡�������Ӱ�죨��1�֣�
��2���ٻ��β����������2�֣�
��ƫС����2�֣� ��ʵ���в��ɱ���������������ʧ��2�֣�
��3������pH��ֽ��������Һ��pH������pH<7��˵������������ˮ�⡣��2�֣�
�ڼ�����Һ����pH��ֽ����Һ��pH������pH�Ȣٵ�pHС��˵������ˮ��Ϊ���ȷ�Ӧ����2�֣�
���������������1����δ����֪Ũ�ȵı�Һ��ϴ�ζ��ܣ��ᵼ�±�ҺŨ��ƫС����Ӧ�����Һ������Ӷ��������ս��ƫ�ߡ�����ƿ�ô���Һ��ϴ��������ƿ�ڲ�������������Һ�����ĵı�Һ��������Ӷ��������ս��ƫ�ߡ��۵ζ�ǰ�����ݣ��ζ������������ݣ����������������Ӷ��������ս��ƫ�ߡ��ܵζ�ǰƽ�ӣ��ζ����˸��ӣ�ʹ�ü��������С���Ӷ��������ս��ƫ�͡�����NaOH�����к�������Na2O,ʹ����õı�ҺŨ��ƫ�����ĵı�Һ�������С���Ӷ��������ս��ƫС��������ʳ��ˮϴ����ƿ����ʵ������Ӱ�졣
��2����ͼ��װ��ȱ�ٻ��β�����������������ձ����粻��Ӳֽ�壬��ᵼ��������ɢʧ���Ӷ�ʹ�ò�õķ�Ӧ��ƫС����ͨ��ʵ���õĦ�H�������������ڣ�57.3kJ��mol��1����˵���䷴Ӧ�ų�������С��57.3kJ��mol��1����ԭ�����ڷ�Ӧ�����в��ɱ���Ĵ�������������ʧ�� ��3��������ˮ��Һ�д��ڣ�Al3++3H2O Al��OH��3+3H+��ͨ��������Һ��pH��˵������������ˮ�⡣�ڼ�����Һ���ɴٽ�ˮ��ij̶ȣ���˿��Լ�����Һ����pH��ֽ����Һ��pH������pH�Ȣٵ�pHС��˵������ˮ��Ϊ���ȷ�Ӧ��
��3��������ˮ��Һ�д��ڣ�Al3++3H2O Al��OH��3+3H+��ͨ��������Һ��pH��˵������������ˮ�⡣�ڼ�����Һ���ɴٽ�ˮ��ij̶ȣ���˿��Լ�����Һ����pH��ֽ����Һ��pH������pH�Ȣٵ�pHС��˵������ˮ��Ϊ���ȷ�Ӧ��
���㣺�к͵ζ��������ˮ��
���������⿼���к͵ζ��в������̶Բⶨ�����Ӱ�죬�к��ȵIJⶨ�������ˮ���֪ʶ����Ŀ�ѶȲ��ɸ�����ѧ֪ʶ���н��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
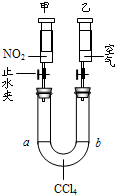 ��1�����й���ʵ���������������ȷ��Ϊ
��1�����й���ʵ���������������ȷ��Ϊ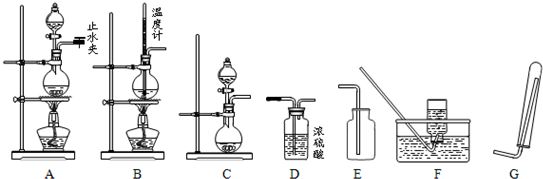
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �ζ����� | ����Һ��� ��mL�� |
��KMnO4��Һ�����mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 25.00 | 0.50 | 20.40 |
| �ڶ��� | 25.00 | 3.00 | 23.00 |
| ������ | 25.00 | 4.00 | 24.10 |
| ������ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ��ɫ | �� | dz�� | �� | ש�� | �� |
| Ksp | 1.77��10-10 | 5.35��10-13 | 1.21��10-16 | 1.12��10-12 | 1.0��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��16�֣���1�����к͵ζ����������У������¸�����������������ʵ�����á�ƫ�ߡ�����ƫ�͡�����Ӱ�족��գ�
�ٵζ���������ˮϴ����δ����֪Ũ�ȵı���Һ��ϴ��ʹ�ζ���� ��
����ƿ������ˮϴ�������ô�����Һ��ϴ��ʹ�ζ���� ��
�۵ζ���(װ����Һ)�ڵζ�ǰ���촦�����ݣ��ζ����������ݣ�ʹ�ζ���� ��
�ܵζ�ǰƽ�ӣ��ζ����˸��ӣ�ʹ�ζ���� ��
���ú�Na2O���ʵ�NaOH������������֪Ũ�ȵı���Һ�����ڵζ�δ֪Ũ�ȵ����ᣬʹ��������Ũ�� ��
��ϴ����ƿʱ�����ϡʳ��ˮ��������ˮ��Ȼ������ƿװ��������ᣬ��NaOH����Һ�ζ�ʱ���Բ�õĽ�� ��
��2����֪H+(aq)+OH-(aq) = H2O(l) ��H= ��57.3kJ��mol��1����50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺

�ٴ�ʵ��װ���Ͽ���ͼ����ȱ��һ�ֲ��������� ��
�ڴ��ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ����ƫС������Ӱ�족)��
����ͨ��ʵ��ⶨ�к��ȵĦ�H�������������ڣ�57.3kJ��mol��1����ԭ������ǣ�
��
��3���ֱ���ƻ�ѧʵ�飬����ѷ���֤����������ˮʱ���������б仯(��ѡ�õ�ҩƷ��������������Һ��ʯ����Һ����̪��Һ��pH��ֽ���ƾ���)��
��֤������������ˮ�ⷴӦ ��
��֤����ˮ�ⷴӦ��һ�����ȷ�Ӧ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com