
| �ζ����� | ������Һ�����mL�� | ������� | |
| �ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 4.20 | 24.00 |
| ������ | 10.00 | 0.40 | 21.50 |
| [(20.50-0.40)+(24.00-4.20)+(21.50-0.40)] |
| 3 |
| [(20.50-0.40)+(24.00-4.20)+(21.50-0.40)] |
| 3 |


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H2��D2��T2 |
| B��14C��14N |
| C��35Cl��37 Cl- |
| D��16O��17O��18O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ζ������У���ƿ������Һ���� |
| B����ƿ������ˮϴ����δ�����T���еζ� |
| C����ʽ�ζ���δ�ñ�������ϴ |
| D���ζ�ǰ��ʽ�ζ��ܼ��첿�������ݣ��ζ���ֹʱ������ʧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����
����һ�������¿��Է�����ͼ��ʾ��ת�������������ˮ����ȥ����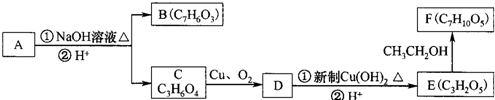
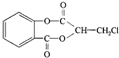
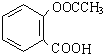 ��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹����
��������ˮ���ᣬ����ʽ C9H8O4�����������������İ�˾ƥ�ֵ�ͬ���칹���� �����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH
�����������л���Ӧ�Ƶã���д��������Ϊ��Ҫԭ���Ʊ��л���C�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�CH3CH2OH | ŨH2SO4 |
| 170�� |
| H2 |
| ����/�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
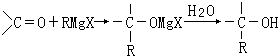
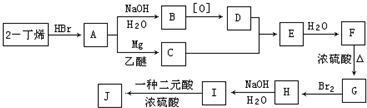
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
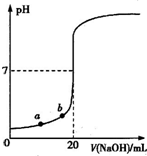
| A��ˮ�������������Ũ�ȣ�a��b |
| B����������ʵ���Ũ��Ϊ0.0100mol?L-1 |
| C��ָʾ����ɫʱ��˵��������NaOHǡ����ȫ��Ӧ |
| D�����μ�NaOH��Һ10.00mLʱ���û��Һ��pH=1+lg3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

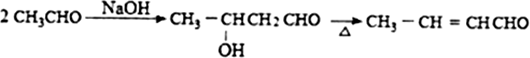
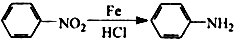 �������а����ױ�������
�������а����ױ��������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com