
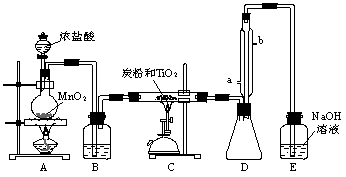
 (�����9��)���Ȼ�������ɫҺ�壬�е�Ϊ136�棬������ˮ�⣬�������е�ˮ���������������̡�(TiCl4��H2O��TiOCl2��2HCl��)��TiCl4����TiO2��Cl2��Ӧ�Ƶ�(TiO2����HCl��Ӧ)���˷�Ӧ��1000������½��еĺ������������̿�۴���ʱ650�桫850�淴Ӧ���˳�����С���ͼ��ijͬѧ��Ƶ�ʵ�����Ʊ�TiCl4��װ�á���ش�
(�����9��)���Ȼ�������ɫҺ�壬�е�Ϊ136�棬������ˮ�⣬�������е�ˮ���������������̡�(TiCl4��H2O��TiOCl2��2HCl��)��TiCl4����TiO2��Cl2��Ӧ�Ƶ�(TiO2����HCl��Ӧ)���˷�Ӧ��1000������½��еĺ������������̿�۴���ʱ650�桫850�淴Ӧ���˳�����С���ͼ��ijͬѧ��Ƶ�ʵ�����Ʊ�TiCl4��װ�á���ش�
(1)Aװ���з�Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
(2)Bװ���е��Լ�Ϊ________________��������______________________________��
(3)ʵ����̿�۵�����Ϊ_______________________��
(4)Dװ��������ˮ��ˮ������Ϊ____________��____________������װ�õ�������_________________��
(5)Eװ����NaOH��Һ��������_______________________________________��
(6)����Ʒ����г���Һ©���е�Ũ�����˳�����¡�E���ܷ�ںͿ��ܵ����⣬����һ�����ԵIJ�����_______________________________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com