
| |||||||||||||||||||||||
(1) |
NH3��H2O |
(2) |
7(�ֱ�ΪCl����NH4+��OH����Na+��NH3��H2O��H2O��H+) |
(3) |
�����𰸣�Cl��,Na+ �����������ڴ˷�Ӧ��Cl����Na+δ���ģ� |
(4) |
�����𰸣�NH4+,NH3��H2O ������������Ӧ����Һ�г�Cl��Ϊ0.01 mol�⣬��Ӧ��ʼʱ��NH4+Ҳ��0.01 mol�����������غ�n(NH4+)��n(NH3��H2O)��0.01 mol�� |
(5) |
�����𰸣�NH4+,H+ ����������NH4Cl��NaOH��Ӧ������0.002 mol NH3��H2O��0.002 mol NaCl����ʣ0.008 mol NH4Cl����NH4+ˮ������ʵ���Ϊx��NH3��H2O�������ʵ���Ϊy���� ������NH4+������H2O ����0.008��x����������������x��������x������0.02��y����������y����y �����ɴ˿���c(NH4+)��c(H+)��c(OH��)��0.008 mol�� |
|
���⿼���й����ӷ�Ӧ�ļ��㣮 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�������������߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��16�֣��ں���������ʵ���Һ�У������ж����ѧƽ��档
��1�������½�0.01 mol NH4Cl��0.002 mol NaOH����ˮ���1L��Һ��
����Һ��Ũ��Ϊ0.01 mol��L-1�������� ��
�����ʵ���֮��Ϊ0.01 mol������������ �� ��
��2�����ʵ���Ũ����ͬ�Ĵ��������������Һ��Ϻ���Һ�д�������Ӻ�������Ũ����ȣ����Ϻ���Һ�� ������ԡ��������ԡ������ԡ������������ ����������Һ��������������������=������
��3����m mol��L-1�Ĵ����n mol��L-1������������Һ�������Ϻ���Һ��pH��7�� m��n�Ĵ�С��ϵ�ǣ� ������������������=����
��4��Ũ��Ϊ0.100 mol��L-1�����и����ʵ���Һ�У�c(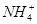 )�ɴ�С��˳����
)�ɴ�С��˳����
��NH4Cl ��NH4HSO4 ��NH3��H2O ��CH3COONH4
��5��ij��Ԫ�ᣨ��ѧʽ��H2A��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�
H2A=H++HA- HA- H++A2-
H++A2-
��֪0.1 mol��L-1��NaHA��ҺpH=2����0.1 mol��L-1��H2A��Һ�������ӵ����ʵ���Ũ��
0.11 mol��L-1(�������=����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и�����ҵ��������飨�������ۻ�ѧ�Ծ��������棩 ���ͣ������
��ͼ��ʾ������֮���ת����ϵ����֪D��E��Z����ѧ��ѧ�����ĵ��ʣ��������ǻ����Z��Y���ȼҵ�IJ�Ʒ��DԪ�ص�ԭ����������������Ӳ�����ȣ���D�������ο�����ˮ����EΪ�ճ�������Ӧ����㷺�Ľ���������Ӧ���⣬������Ӧ����ˮ��Һ�н��С���ش��������⡣
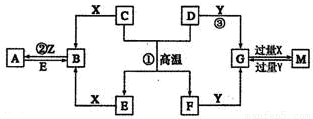
��1��д��X�ĵ���ʽ��??????????? ��
��2������ʱpH =12��G��Һ�У����ʵ������������ʵ�������Ũ��֮��Ϊ?????????? ��д������ʽ����
��3���ڵ�ƹ�ҵ�У�����E��Ϊ���ƽ�����ͭΪ�Ʋ��������ͭ��____������д����E�缫�Ϸ����ĵ缫��Ӧʽ��????????????? ��
��4��д����Ӧ�������ӷ���ʽ��??????????????????? ��
��5�������ޱ�ǩ��Y��M��������ɫˮ��Һ�����������κ��Լ����������һ����ʵ�����������Һ���Լ���???????????????? ���ش�ʵ������������ۣ���
��6��A��Һ��NaOH��Һ��Ͽ��γɳ�����ij�¶��´˳�����Ksp =2.097��l0��39����0.01 mol/L��A��Һ��0.001 mol��L��1��NaOH��Һ�������ϣ�����Ϊ�ܷ��γɳ���____��������������������������ͨ������˵��????????????????????????????? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��1��������pH=5 HCl��Һ��pH=5��AlCl3��Һ����ˮ���������c (H+) ֮�ȵ��� ��
��2��д����ĭ��������ʱ������Ӧ�����ӷ���ʽ ��
��3�������½�0.01molCH3COONa��0.02mol��������ˮ�����0.5L�����Һ����Һ�й��� ������������Ũ�ȴӴ�С��˳��Ϊ ��
��4�������£���100 mL 0.01 mol��L��1HA��Һ��μ���0.02 mol��L��1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯�����������Һ���ʱ������仯����
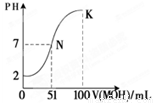
�ش��������⣺
����ͼ����Ϣ��֪HAΪ_______�ᣨ�ǿ���������� �� K���Ӧ����Һ��c(M+)��c(MOH)= mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�������������߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��16�֣��ں���������ʵ���Һ�У������ж����ѧƽ��档
��1�������½�0.01 mol NH4Cl��0.002 mol NaOH����ˮ���1L��Һ��
����Һ��Ũ��Ϊ0.01 mol��L-1�������� ��
�����ʵ���֮��Ϊ0.01 mol������������ �� ��
��2�����ʵ���Ũ����ͬ�Ĵ��������������Һ��Ϻ���Һ�д�������Ӻ�������Ũ����ȣ����Ϻ���Һ�� ������ԡ��������ԡ������ԡ��� ��������� ����������Һ��������������������=������
��3����m mol��L-1�Ĵ����n mol��L-1������������Һ�������Ϻ���Һ��pH��7�� m��n�Ĵ�С��ϵ�ǣ� ������������������=����
��4��Ũ��Ϊ0.100 mol��L-1�����и����ʵ���Һ�У�c(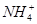 )�ɴ�С��˳����
)�ɴ�С��˳����
��NH4Cl ��NH4HSO4 ��NH3��H2O ��CH3COONH4
��5��ij��Ԫ�ᣨ��ѧʽ��H2A��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�
H2A=H++HA-
HA- H++A2-
H++A2-
��֪0.1 mol��L-1��NaHA��ҺpH=2����0.1 mol��L-1��H2A��Һ�������ӵ����ʵ���Ũ��
0.11 mol��L-1(�������=����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������
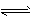 2NH3 ( g) ��H = -a kJ . mol-1
2NH3 ( g) ��H = -a kJ . mol-1  NH3(g) ��H = -b kJ . mol-1
NH3(g) ��H = -b kJ . mol-1 �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com