
���� ��1����ͭ��̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1��
��2��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���Ԫ��2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܴ���ͬ��������Ԫ�صģ��ʵ�һ������F��N��O��C��B��
��3��Cu�ǽ��������ڽ������壬NH4F���Σ��������Ӿ��壬NH3��F2��NF3�����ڷ��Ӿ��壻
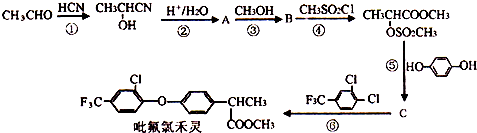
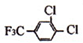
��4������Q�Ľṹ��֪��Q�����д�������������ۼ�����λ����O��B֮�䣩�����Ӽ���������
��R��������ΪH3+O������ԭ����ԭ�Ӽ۲���Ӷ���=3+$\frac{6-1��3-1}{2}$=4���µ��Ӷ�=$\frac{6-1��3-1}{2}$=1���ݴ��жϣ�
��5����Cu���ʵķ�ĩ����NH3��Ũ��Һ�У�ͨ��O2����ַ�Ӧ����Һ������ɫ��˵����Ӧ������[Cu��NH3��4]2+���÷�Ӧ�����ӷ���ʽΪ��2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
��6����������������ÿ��Feԭ����Χ��8��Feԭ�ӣ�Fe���ʵľ����ṹΪ����������������Feԭ����ĿΪ��1+8��$\frac{1}{8}$=2�������ӵ�����ΪNA��Feԭ�ӵ�Ħ������Ϊ56g/mol���ʾ�������Ϊ$\frac{2��56}{{N}_{A}}$����Feԭ�Ӱ뾶Ϊx���辧���ⳤΪy����������Խ���Ϊ4x����4x��2=y2+y2+y2����y=$\frac{4\sqrt{3}}{3}$x���������Ϊ��V=y3=��$\frac{4\sqrt{3}}{3}$x��3������þ������ܶ�Ϊ��g/cm3��������=��$\frac{4\sqrt{3}}{3}$x��3����=$\frac{2��56}{{N}_{A}}$���ɴ˽��
��� �⣺��1����ͭ��̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1��δ�ɶԵ�����ĿΪ1���ʴ�Ϊ��1��
��2��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���Ԫ��2p�ܼ�Ϊ�����ȶ�״̬����һ�����ܴ���ͬ��������Ԫ�صģ��ʵ�һ������F��N��O��C��B��C��N��O����Ԫ�ص����λ��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��Cu�ǽ��������ڽ������壬NH4F���Σ��������Ӿ��壬NH3��F2��NF3�����ڷ��Ӿ��壬
�ʴ�Ϊ�����Ӿ��塢���Ӿ��塢�������壻
��4������Q�Ľṹ��֪��Q�����д�������������ۼ�����λ����O��B֮�䣩�����Ӽ���������
�ʴ�Ϊ��bcef��
��R��������ΪH3+O������ԭ����ԭ�Ӽ۲���Ӷ���=3+$\frac{6-1��3-1}{2}$=4���µ��Ӷ�=$\frac{6-1��3-1}{2}$=1��Ϊ�����ͣ���ԭ�Ӳ�ȡsp3�ӻ���
�ʴ�Ϊ�������ͣ�3��2p��
��5����Cu���ʵķ�ĩ����NH3��Ũ��Һ�У�ͨ��O2����ַ�Ӧ����Һ������ɫ��˵����Ӧ������[Cu��NH3��4]2+���÷�Ӧ�����ӷ���ʽΪ��2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
�ʴ�Ϊ��2Cu+8NH3•H2O+O2=2[Cu��NH3��4]2++4OH-+6H2O��
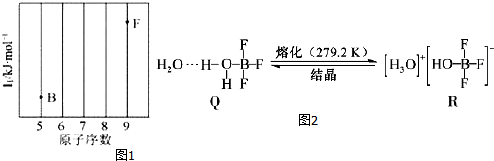
��6��ͼʾFe�ľ���Ϊ���������ѻ���ʽ������λ��Ϊ8��
Fe���ʵľ����ṹΪ����������������Feԭ����ĿΪ��1+8��$\frac{1}{8}$=2�������ӵ�����ΪNA��Feԭ�ӵ�Ħ������Ϊ56g/mol���ʾ�������Ϊ$\frac{2��56}{{N}_{A}}$����Feԭ�Ӱ뾶Ϊx���辧���ⳤΪy����������Խ���Ϊ4x����4x��2=y2+y2+y2����y=$\frac{4\sqrt{3}}{3}$x���������Ϊ��V=y3=��$\frac{4\sqrt{3}}{3}$x��3������þ������ܶ�Ϊ��g/cm3��������=��$\frac{4\sqrt{3}}{3}$x��3����=$\frac{2��56}{{N}_{A}}$�������ã�x=$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{2��56}{{N}_{A}}}$��cm����
�ʴ�Ϊ��8��$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{2��56}{{N}_{A}}}$��
���� ���⿼�����ʽṹ�����ʣ��漰��������Ų����ɡ���һ�����ܡ��ӻ����ۡ��������㣬�Ѷ��еȣ���6���м���Ϊ�״��㡢�ѵ㣬���ݾ���ṹȷ��ԭ�Ӱ뾶�뾧���ⳤ��ϵ�ǹؼ���ע�����þ�̯�����о�����ԭ����Ŀ���㣮


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 25�棬1.01��105Pa��64g SO2�к��е�ԭ����Ϊ3NA | |
| B�� | ����Fe��1 mol Cl2��Ӧ��ת����3NA������ | |
| C�� | ����ȼ�ϵ����������22.4 L����״��������ʱ����·��ͨ���ĵ�����ĿΪ2NA | |
| D�� | ��״���£�11.2L H2O���еķ�����Ϊ0.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �����Ӱ뾶�Ĵ�С˳��g��h��e��f | |
| B�� | ��x �γɻ�����ķе㣺d��z��y | |
| C�� | zh3�и�ԭ�Ӿ�����8���ӽṹ | |
| D�� | x��y��z��d����Ԫ�ظ�����ۺ���ͼ۴����ͷֱ�Ϊ0��0��2��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 +
+ $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$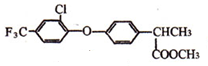 +HCl��
+HCl�� ��
�� �Ʊ��߷��ӻ�����
�Ʊ��߷��ӻ�����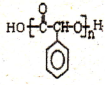 �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�����ʾ��ͼ���£�CH3CH2OH$��_{170��}^{H_{2}SO_{4}}$CH2�TCH2$��_{������}^{H_{2}}$CH3CH3��
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�����ʾ��ͼ���£�CH3CH2OH$��_{170��}^{H_{2}SO_{4}}$CH2�TCH2$��_{������}^{H_{2}}$CH3CH3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 60g�����д��ڵĹ��ۼ�����Ϊ10NA | |
| B�� | 50mL12mol•L-1����������MnO2���ȣ�ת�Ƶĵ�����Ϊ0.3NA | |
| C�� | 5NH4NO3$\frac{\underline{\;\;��\;\;}}{\;}$2HNO3+4N2��+9H2O��Ӧ�У�����28gN2ʱ��ת�Ƶĵ�����Ϊ3.75NA | |
| D�� | 235g����$\stackrel{239}{92}$U�����ѱ䷴Ӧ��$\stackrel{239}{92}$ U+$\stackrel{1}{0}$n$\stackrel{�ѱ�}{��}$$\stackrel{90}{38}$Sr+$\stackrel{136}{54}$U+10$\stackrel{1}{0}$n�������������ӣ�$\stackrel{1}{0}$n����Ϊ10 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ܱ�������2molNO��1molO2��ַ�Ӧ������ķ�����Ϊ2NA | |
| B�� | һ���¶��£�1L0.50mol/LNH4NO3��Һ�к���ԭ�Ӹ���ΪNA | |
| C�� | ��������ֽ��Ƶñ�״����1.12LO2��ת�Ƶ�����ĿΪ0.2NA | |
| D�� | 28g����ʽΪCnH2n�������к��е�C-H������ĿΪ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
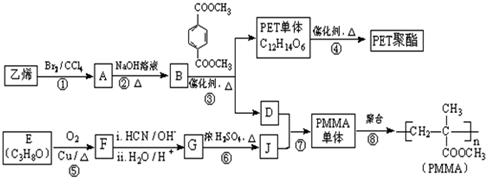
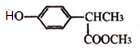
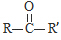 $\underset{\stackrel{i��HCN/O{H}^{-}}{��}}{ii��{H}_{2}O/{H}^{+}}$
$\underset{\stackrel{i��HCN/O{H}^{-}}{��}}{ii��{H}_{2}O/{H}^{+}}$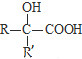 ��R��R�����������
��R��R�����������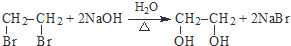 ��
��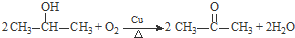 ��
�� ��
��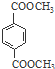 ������NaOH��Һ��Ӧʱ���������4mol NaOH
������NaOH��Һ��Ӧʱ���������4mol NaOH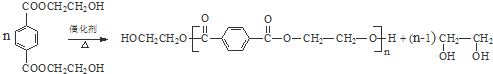 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �ᴿ���������������������������������������������м������NaOH��Һ�����÷�Һ | |
| B�� | ��ȥ����CO2�л��е�����SO2���������������ͨ��ʢ������KMnO4��Һ��Ũ�����ϴ��ƿ | |
| C�� | ̽����ͬ������H2O2�ֽ����ʵ�Ӱ�죺����ͬ�����£�����֧�Թܾ�����2 mL5%H2O2��Ȼ��ͬʱ�ֱ������������MnO2�����ĩ��FeCl3�����ĩ���۲첢�Ƚ�ʵ������ | |
| D�� | ֤�����Ľ����Ա�ͭǿ�������£��ֱ���ʢ��Ũ�������֧�Թ��м������ۺ�ͭ�ۣ��۲첢�Ƚϲ�������Ŀ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com