
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
(4)֤��NaHSO3��Һ��HSO �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
a���ⶨ��Һ��pH
b������Ba(OH)2��Һ
c����������
d������Ʒ����Һ
e������ɫʯ����ֽ���
(5)����Na2S2O5�����ڿ������ѱ�������ʵ�鷽����__________��
(5)ȡ����Na2S2O5�������Թ��У�������ˮ�ܽ⣬�μ��������ᣬ���ٵ����Ȼ�����Һ���а�ɫ��������
 �߽�������ϵ�д�
�߽�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���У���ȷ����(����)
A.�����ڱ������Ԫ�����ڵ�����������ԭ�Ӻ��������
B.�����ڱ��Ԫ�����ڵ�����������ԭ�Ӻ�����Ӳ���
C.����������Ϊ8�Ķ���ϡ������Ԫ�ص�ԭ��
D.Ԫ�ص�ԭ������Խ����ԭ�Ӱ뾶ҲԽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵķ����м��ЦҼ������Цм�����(����)
��HCl����H2O����N2����H2O2����C2H4����C2H2
A���٢ڢ� B���ۢܢݢ�
C���٢ۢ� D���ۢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ϊ����CH3COONa��Һ�м������������ᣬ����CH3COONa��Һˮ�������OH����Ӧ��ʹƽ����ˮ�ⷽ���ƶ�������˵������Ϊʲô��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ɫ����Һ���ܴ����������������(����)
A��Na����Al3����HCO ��NO
��NO
B��AlO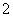 ��Cl����Mg2����K��
��Cl����Mg2����K��
C��NH ��Na����CH3COO����NO
��Na����CH3COO����NO
D��Na����NO ��Cl����I��
��Cl����I��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�˳�ȥMgCl2��Һ�е�FeCl3�����ڼ��Ƚ���������¼���һ���Լ������Լ���(����)
A��NaOH B��Na2CO3 C����ˮ D��MgO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���Ũ�Ⱦ�Ϊ1 mol·L��1������������Һ��
��H2SO4��Һ����NaHCO3��Һ����NH4Cl��Һ
��NaOH��Һ
(1)��������ҺpH�ɴ�С��˳����______��������ˮ�����H��Ũ����С����______��(�������)
(2)���и�����Ũ���ɴ�С��˳����____________��NaHCO3��ˮ��ƽ�ⳣ��Kh��______mol·L��1��(��֪̼��ĵ��볣��K1��4��10��7��K2��5.6��10��11)
(3)�����ͨ��������������ʱ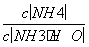 ��ֵ______(���������С�����䡱)��
��ֵ______(���������С�����䡱)��
(4)�����ۺܻ͢�Ϻ���Һǡ�ó����ԣ�����ǰ�۵����______�ܵ����(����ڡ�����С�ڡ����ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ӷ���ʽ��д
(1)[2014·�¿α�ȫ������27(1)]H3PO2��һԪ��ǿ�ᣬд������뷽��ʽ________________________________________________________________________��
(2)[2014·���ȫ������27(1)��ѡ]��֪�ǽ���Ԫ��A����������������������ͬ����A��F��ȼ�գ���������ˮ�õ�һ��ǿ��(ע��A��F��Ϊ������Ԫ��)��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ
________________________________________________________________________��
(3)[2014·������ۣ�9(4)�ı�]��֪Na2S2O3�����Ի�����������ȶ��������������»��ƣ������ӷ���ʽ��ʾ��Ƶ�ԭ��________________________________________________________________________��
(4)[2014·ɽ�����ۣ�30(3)��ѡ]ȡ�������ۺ�Fe2O3�����ȷ�Ӧ�����õĹ������������������ϡH2SO4���μ�KSCN��Һ����������________(��ܡ����ܡ�)˵��������������Fe2O3��������________________________________________________________________________
(�����ӷ���ʽ˵��)��
(5)[2014·�Ĵ����ۣ�8(4)�ı�]��ͭ���ʵķ�ĩ����NH3��Ũ��Һ�У�ͨ��O2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ȶ���M������ĵ��ߵ��²��ϣ���ҵ�Ͽ����л�����ԭ��A��E�Ƶã���ϳ�·������ͼ��ʾ��
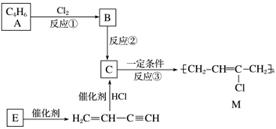
��֪��H2CCHCCH��E���۵õ�
���������գ�
(1)A��������________________����Ӧ�۵ķ�Ӧ������________��
(2)д����Ӧ�ڵĻ�ѧ��Ӧ����ʽ_________________________________________________��
(3)Ϊ�о����ʵķ����ԣ���E���ۡ��ľ۳ɻ�״�����д�����ǵĽṹ��ʽ��_____________________________________________________________________��
������������״��������Լ�Ϊ_____________________________________________��
(4)��������A�Ʊ���������PB��ԭ��֮һ1,4������(BDO)�ĺϳ�·�ߣ�

д��������A�Ʊ�BDO�Ļ�ѧ��Ӧ����ʽ��
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com