
��14�֣���ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ�� 2CH3OH+3O2+4KOH 2K2CO3+6H2O ��д���пհף�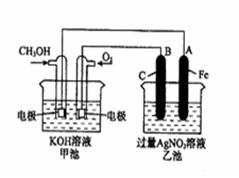
��1����д���ס������ص����ơ������ ���ҳ��� ��
��2���׳���ͨ��CH3OH�ĵ缫������ ���缫��Ӧ����ʽΪ ���ҳ���B��ʯī���缫�������� ��
��3���������У��ҳ���ҺpH�ı仯Ϊ�������ߡ��������͡����䡱 �� ��
��4�����ҳ���A��Fe��������������5.40gʱ���׳�������������O2 mL����״���£�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣���ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��
2CH3OH+3O2+4KOH 2K2CO3+6H2O
��1����ش�
����� װ�ã�B��ʯī���缫�������� ��
��2��д�����е缫��Ӧʽ��
ͨ��CH3OH �ĵ缫�ĵ缫��Ӧʽ�ǣ� ��
B��ʯī���缫�ĵ缫��ӦʽΪ�� ��
��3���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
��4�����ҳ���A��Fe��������������5.40gʱ���׳���������ת�Ƶ��� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡΫ���и߶���ѧ����ĩͳ����ѧ�Ծ��������棩 ���ͣ������
��ͼ��һ����ѧ���̵�ʾ��ͼ���ش��������⣺
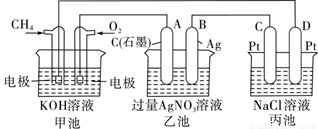
��1���׳��� װ�ã��缫A�������� ��
��2����װ����ͨ��CH4�ĵ缫��ӦʽΪ ����װ����B(Ag)�ĵ缫��ӦʽΪ ����װ����D���IJ����� ��д��ѧʽ����
��3��һ��ʱ�䣬�������в���112mL����״���£�����ʱ�����Ƚ�����أ�������Һ��25�� ʱ��pH =__________������֪��NaCl��Һ������������Һ���Ϊ500 mL����
��Ҫʹ���ػָ����ǰ��״̬��Ӧ�������ͨ�� ��д��ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�����и߶���ѧ����ĩ�������ƻ�ѧ���������棩 ���ͣ������
��16�֣���ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��
2CH3OH+3O2+4KOH 2K2CO3+6H2O

��1����ش�ͼ�мס������ص����ơ��׳��� װ�ã��ҳ��� װ�á�
��2����ش����е缫�����ƣ�ͨ��CH3OH�ĵ缫������ ��B��ʯī���缫�������� ��
��3��д���缫��Ӧʽ�� ͨ��O2�ĵ缫�ĵ缫��Ӧʽ��
A��Fe���缫�ĵ缫��ӦʽΪ
��4���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
��5�����ҳ���A��Fe��������������5.40gʱ���׳�������������O2 mL����״���£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ʡ������ĩ���Ի�ѧ�Ծ� ���ͣ������
��9�֣���ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��
2CH3OH+3O2+4KOH
2K2CO3+6H2O
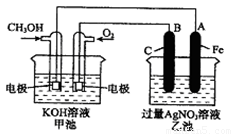
��1����ش�
����� װ�ã�B��ʯī���缫�������� ��
��2��д�����е缫��Ӧʽ��
ͨ��CH3OH �ĵ缫�ĵ缫��Ӧʽ�ǣ� ��
B��ʯī���缫�ĵ缫��ӦʽΪ�� ��
��3���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
��4�����ҳ���A��Fe��������������5.40gʱ���׳���������ת�Ƶ��� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�곤�������ѧУ�߶���һѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��14�֣���ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ�� 2CH3OH+3O2+4KOH 2K2CO3+6H2O
��д���пհף�
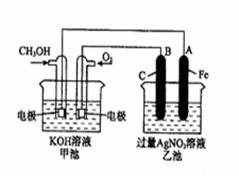
��1����д���ס������ص����ơ������ ���ҳ��� ��
��2���׳���ͨ��CH3OH�ĵ缫������ ���缫��Ӧ����ʽΪ ���ҳ���B��ʯī���缫�������� ��
��3���������У��ҳ���ҺpH�ı仯Ϊ�������ߡ��������͡����䡱 �� ��
��4�����ҳ���A��Fe��������������5.40gʱ���׳�������������O2 mL����״���£�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com