
ŹµŃéŹŅÓŠŅ»Ęæ»ģÓŠĀČ»ÆÄʵÄĒāŃõ»ÆÄĘ¹ĢĢåŹŌ¼Į£¬¾²ā¶ØĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹżŌ¼ĪŖ82%£¬ĪŖĮĖŃéÖ¤Ęä“æ¶Č£¬ÓĆÅضČĪŖ0.2 mol”¤L£1µÄŃĪĖį½ųŠŠµĪ¶Ø£¬Ķź³ÉĻĀĮŠĪŹĢā£ŗ
(1)³ĘČ”5.0 gøĆĒāŃõ»ÆÄĘ¹ĢĢåѳʷ£¬Åä³É500 mLČÜŅŗ±øÓĆ”£
(2)½«±ź×¼ŃĪĖį×°ŌŚ25.00 mLµÄ________µĪ¶Ø¹ÜÖŠ£¬µ÷½ŚŅŗĆęĪ»ÖĆŌŚ”°0”±æĢ¶ČŅŌĻĀ£¬²¢¼ĒĀ¼ĻĀæĢ¶Č”£
(3)Č”20.00 mL“ż²āŅŗ”£øĆĻīŹµŃé²Ł×÷Ź¹ÓƵÄÖ÷ŅŖŅĒĘ÷ÓŠ________”£ÓĆ·ÓĢŖ×÷ÖøŹ¾¼ĮŹ±£¬µĪ¶Øµ½ČÜŅŗŃÕÉ«ÓÉ________É«øÕŗƱä³É________É«ĪŖÖ¹”£
(4)µĪ¶Ø“ļÖÕµćŗ󣬼ĒĻĀŃĪĖįÓĆČ„20.00 mL£¬¼ĘĖćĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹżĪŖ________”£
(5)ŹŌ·ÖĪöÉĻŹöµĪ¶ØĪó²īæÉÄÜÓÉĻĀĮŠÄÄŠ©ŹµŃé²Ł×÷ŅżĘš________(ĢīŠņŗÅ)”£
| A£®×ŖŅĘ“ż²āŅŗÖĮČŻĮæĘæŹ±£¬Ī“Ļ“µÓÉÕ± |
| B£®ĖįŹ½µĪ¶Ø¹ÜÓĆÕōĮóĖ®Ļ“µÓŗó£¬Ö±½Ó×°ŃĪĖį |
| C£®µĪ¶ØŹ±·“Ó¦Ę÷Ņ”¶ÆĢ«¼¤ĮŅ£¬ÓŠÉŁĮæŅŗĢ彦³ö |
| D£®µĪ¶Øµ½ÖÕµćŹ±£¬µĪ¶Ø¹Ü¼ā×ģŠüÓŠĘųÅŻ |
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ¹ŲÓŚ0.1 mol”¤L£1CH3COONaČÜŅŗµÄĖµ·ØÕżČ·µÄŹĒ £Ø £©
| A£®¼ÓČėÉŁĮæNaOH¹ĢĢ壬c(CH3COO£)Ōö“ó |
| B£®¼ÓČėÉŁĮæFeCl3¹ĢĢ壬c(CH3COO£)Ōö“ó |
| C£®Ļ”ŹĶČÜŅŗ£¬ČÜŅŗµÄpHŌö“ó |
| D£®¼ÓČėŹŹĮæ“×Ėį£¬µĆµ½µÄĖįŠŌ»ģŗĻČÜŅŗ£ŗc(Na£«)£¾c(CH3COO£) |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©Õą¼°ĘäijŠ©»ÆŗĻĪļŹĒµ¼µÆ»š¼żÖʵ¼ÖŠµÄÖŲŅŖ¹āµē²ÄĮĻ£¬¹¤ŅµÉĻÉś²śÕąµÄĮ÷³ĢČēĻĀ£ŗ
ŅŃÖŖ£ŗGeO2ÓėÅØŃĪĖį·“Ӧɜ³ÉĖÄĀČ»ÆÕą£ØČŪµćĪŖ”Ŗ51.5”ę£¬·ŠµćĪŖ86.6”ę£©”£ĖÄĀČ»ÆÕąŅ×Ė®½ā”£
£Ø1£©²½Öč¢ŚÖŠÅØŃĪĖį³żĮĖ×÷ĪŖ·“Ó¦ĪļĶā£¬»¹ÓŠŅ»øöÖŲŅŖ×÷ÓĆŹĒ ”£
£Ø2£©²½Öč¢ŪæŲÖʵÄĪĀ¶Č·¶Ī§ ”£
£Ø3£©Š“³ö·“Ó¦µÄ¢Ü”¢¢ŽµÄ»Æѧ·½³ĢŹ½
¢Ü £»¢Ž ”£
£Ø4£©Éś²ś¹ż³ĢÖŠæÉŅŌŃ»·ĄūÓƵÄĪļÖŹŹĒ £ØĢī»ÆѧŹ½£©
£Ø5£©²½Öč¢ŁÖŠ²śÉśµÄSO2æÉŅŌÓĆ¼īŅŗĪüŹÕ£¬ĒėŠ“³öĄė×Ó·“Ó¦·½³ĢŹ½ ”£
£Ø6£©Ēė¼ņŅŖĆčŹöŹµŃéŹŅÖŠ²Ł×÷aµÄ¹ż³Ģ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
pCĄąĖĘpH,ŹĒÖø¼«Ļ”ČÜŅŗÖŠ,ČÜÖŹĪļÖŹµÄĮæÅØ¶ČµÄ³£ÓƶŌŹżøŗÖµ”£ČēijČÜŅŗČÜÖŹµÄÅضČĪŖ1”Į10-3mol”¤L-1,ŌņøĆČÜŅŗÖŠČÜÖŹµÄpC=-lg(1”Į10-3)=3”£ĻĀĶ¼ĪŖ,H2CO3ŌŚ¼ÓČėĒæĖį»ņĒæ¼īČÜŅŗŗó,Ę½ŗāŹ±ČÜŅŗÖŠČżÖֳɷֵÄpC”ŖpHĶ¼”£Ēė»Ų“šĻĀĮŠĪŹĢā: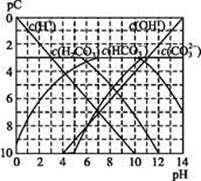
(1)ŌŚČĖĢåŃŖŅŗÖŠ,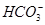 ÄÜĘšµ½Ź¹ČĖĢåŃŖŅŗpH±£³ÖŌŚ7.35~7.45µÄ×÷ÓĆ”£¢ŁĒėÓƵē½āÖŹČÜŅŗÖŠµÄĘ½ŗā½āŹĶ:””””””””””””””””””””””””””””””(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)”£
ÄÜĘšµ½Ź¹ČĖĢåŃŖŅŗpH±£³ÖŌŚ7.35~7.45µÄ×÷ÓĆ”£¢ŁĒėÓƵē½āÖŹČÜŅŗÖŠµÄĘ½ŗā½āŹĶ:””””””””””””””””””””””””””””””(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)”£
¢ŚÕż³£ČĖĢåŃŖŅŗÖŠ,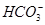 µÄĖ®½ā³Ģ¶Č””””””””µēĄė³Ģ¶Č(Ģī”°<”±”°>”±»ņ”°=”±)”£
µÄĖ®½ā³Ģ¶Č””””””””µēĄė³Ģ¶Č(Ģī”°<”±”°>”±»ņ”°=”±)”£
¢ŪpH=7.00µÄŃŖŅŗÖŠ,c(H2CO3)””””””””c(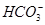 )(Ģī”°<”±”°>”±»ņ”°=”±)”£
)(Ģī”°<”±”°>”±»ņ”°=”±)”£
(2)H2CO3Ņ»¼¶µēĄėĘ½ŗā³£ŹżµÄŹżÖµ ”Ö”””””””””£
”Ö”””””””””£
(3)ijĶ¬Ń§ČĻĪŖøĆČÜŅŗÖŠNa2CO3µÄĖ®½āŹĒĪ¢ČõµÄ,·¢ÉśĖ®½āµÄC ²»³¬¹żĘä×ÜĮæµÄ10%”£ĒėÄćÉč¼Ę¼ņµ„ŹµŃéÖ¤Ć÷øĆĶ¬Ń§µÄ¹ŪµćŹĒ·ńÕżČ·”£
²»³¬¹żĘä×ÜĮæµÄ10%”£ĒėÄćÉč¼Ę¼ņµ„ŹµŃéÖ¤Ć÷øĆĶ¬Ń§µÄ¹ŪµćŹĒ·ńÕżČ·”£
(4)ŅŃÖŖijĪĀ¶ČĻĀLi2CO3µÄKspĪŖ1.68”Į10-3,½«ŹŹĮæLi2CO3¹ĢĢåČÜÓŚ100 mLĖ®ÖŠÖĮøÕŗƱ„ŗĶ,±„ŗĶLi2CO3ČÜŅŗÖŠc(Li+)="0.15" mol”¤L-1.c( )="0.075" mol”¤L-1”£Čōt1Ź±æĢŌŚÉĻŹöĢåĻµÖŠ¼ÓČė100 mL 0.125 mol”¤L-1 Na2CO3ČÜŅŗ,ĮŠŹ½¼ĘĖćĖµĆ÷ŹĒ·ńÓŠ³Įµķ²śÉś”£
)="0.075" mol”¤L-1”£Čōt1Ź±æĢŌŚÉĻŹöĢåĻµÖŠ¼ÓČė100 mL 0.125 mol”¤L-1 Na2CO3ČÜŅŗ,ĮŠŹ½¼ĘĖćĖµĆ÷ŹĒ·ńÓŠ³Įµķ²śÉś”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
³£ĪĀĻĀ£¬½«Ä³Ņ»ŌŖĖįHAŗĶNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬Į½ÖÖČÜŅŗµÄÅضČŗĶ»ģŗĻŗóĖłµĆČÜŅŗµÄpHČēĻĀ±ķ£ŗ
| ŹµŃ鱹ŗÅ | HA | NaOH | »ģŗĻČÜŅŗµÄpH |
| ¼× | [HA]£½0.2 mol”¤L£1 | [NaOH]£½0.2 mol”¤L£1 | pH£½a |
| ŅŅ | [HA]£½c1 mol”¤L£1 | [NaOH]£½0.2 mol”¤L£1 | pH£½7 |
| ±ū | [HA]£½0.1 mol”¤L£1 | [NaOH]£½0.1 mol”¤L£1 | pH£½9 |
| ¶” | pH£½2 | pH£½12 | pH£½b |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(1)ÅØ¶Č¾łĪŖ0.01 mol”¤L-1µÄ8ÖÖČÜŅŗ:¢ŁHNO3””¢ŚH2SO4””¢ŪCH3COOH””¢ÜBa(OH)2””¢ŻNaOH
¢ŽCH3COONa””¢ßKCl””¢ąNH4Cl,ÕāŠ©ČÜŅŗpHÓÉŠ”µ½“óµÄĖ³ŠņŹĒ(ĢīŠ“±ąŗÅ)”””””””””””””””””””£
(2)pH=2µÄijĖįHnA(An+ĪŖĖįøł)ÓėpH=12µÄij¼īB(OH)m»ģŗĻ,Ē”ŗĆ·“Ӧɜ³ÉÕżŃĪ,»ģŗĻŅŗpH=8”£
¢Ł·“Ӧɜ³ÉµÄÕżŃĪµÄ»ÆѧŹ½ĪŖ”””””””””””””””””£
¢ŚøĆŃĪÖŠ””””””””Ąė×ÓŅ»¶ØÄÜĖ®½ā,Ę䵌Ņ»²½Ė®½āµÄĄė×Ó·½³ĢŹ½ĪŖ”””””””””””””””””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖČÜŅŗÖŠÖ»“ęŌŚOH£”¢H£«”¢Na£«”¢CH3COO£ĖÄÖÖĄė×Ó£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ČōČÜŅŗÖŠÖ»ČܽāĮĖŅ»ÖÖČÜÖŹ£¬ŌņøĆČÜÖŹŹĒ £¬ČÜŅŗÖŠc(H£«£© c(OH£)(Ģī”°£¾”±”¢”°£½”±»ņ”°£¼”±)”£
£Ø2£©ČōČÜŅŗÖŠc(Na£«)£¾c(OH£)£¾c(CH3COO£)£¾c(H£«)£¬ŌņøĆČÜŅŗÖŠµÄČÜÖŹĪŖ £¬ČōČÜŅŗÖŠc(CH3COO£)£¾c(Na£«)£¾c(H£«)£¾c(OH£)£¬ŌņøĆČÜŅŗÖŠČÜÖŹĪŖ ”£
£Ø3£©ČōøĆČÜŅŗŹĒÓÉĢå»żĻąµČµÄNaOHČÜŅŗŗĶ“×ĖįČÜŅŗ»ģŗĻ¶ų³É£¬ĒŅĒ”ŗĆ³ŹÖŠŠŌ£¬Ōņ»ģŗĻĒ°c(NaOH£© c(CH3COOH)£¬»ģŗĻĒ°¼īÖŠc(OH£)ŗĶĖįÖŠc(H£«)µÄ¹ŲĻµc(OH££© c(H£«)(Ģī”°£¾”±£¬”°£½”±»ņ”°£¼”±)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Į¶½š·ĻĖ®ÖŠŗ¬ÓŠĀēĄė×Ó[Au(CN)2]££¬ĘäµēĄė³öµÄCN£ÓŠ¶¾£¬µ±CN£ÓėH£«½įŗĻÉś³ÉHCNŹ±£¬¶¾ŠŌøüĒ攣»Ų“šĻĀĮŠĪŹĢā”£
(1)¾ē¶¾ĪļHCNµÄĖ®ČÜŅŗĖįŠŌŗÜČõ£¬Š“³öĘäµēĄėµÄ·½³ĢŹ½£ŗ ”£
(2)ÓėČõµē½āÖŹĢ¼ĖįµÄµēĄė·½Ź½ĻąĖĘ£¬[Au(CN)2]£Ņ²“ęŌŚ×ÅĮ½²½µēĄė£¬ĘäŅ»¼¶µēĄė·½³ĢŹ½ĪŖ ”£
(3)“¦ĄķÕāÖÖ·ĻĖ®ŹĒŌŚ¼īŠŌĢõ¼žĻĀ£¬ĄūÓĆNaClO½«CN£Ńõ»ÆĪŖCO32-ŗĶN2£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ ”£ŌŚĖįŠŌĢõ¼žĻĀ£¬ClO£Ņ²ÄÜŃõ»ÆCN££¬µ«Źµ¼Ź“¦Ąķ·ĻĖ®Ź±Č“²»ŌŚĖįŠŌĢõ¼žĻĀ½ųŠŠµÄÖ÷ŅŖŌŅņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»ÆѧѧæĘÖŠµÄ»ÆŃ§Ę½ŗā”¢µēĄėĘ½ŗā”¢Ė®½āĘ½ŗāŗĶČܽāĘ½ŗā¾ł·ūŗĻĄÕĻÄĢŲĮŠŌĄķ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©³£ĪĀĻĀ£¬Č”pH£½2µÄŃĪĖįŗĶ“×ĖįČÜŅŗø÷100 mL£¬ĻņĘäÖŠ·Ö±š¼ÓČėŹŹĮæµÄZnĮ££¬·“Ó¦¹ż³ĢÖŠĮ½ČÜŅŗµÄpH±ä»ÆČēĶ¼ĖłŹ¾”£ŌņĶ¼ÖŠ±ķŹ¾“×ĖįČÜŅŗÖŠpH±ä»ÆĒśĻߵďĒ________£ØĢī”°A”±»ņ”°B”±£©”£ÉčŃĪĖįÖŠ²Ī¼Ó·“Ó¦µÄZnĮ£ÖŹĮæĪŖm1£¬“×ĖįČÜŅŗÖŠ²Ī¼Ó·“Ó¦µÄZnĮ£ÖŹĮæĪŖm2£¬Ōņm1________m2£ØŃ”Ģī”°£¼”±”¢”°£½”±»ņ”°£¾”±£©”£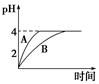
£Ø2£©ŅŃÖŖ³£ĪĀĻĀCu£ØOH£©2µÄKsp£½2”Į10£20”£ÓÖÖŖ³£ĪĀĻĀijCuSO4ČÜŅŗĄļc£ØCu2£«£©£½0.02 mol”¤L£1£¬Čē¹ūŅŖÉś³ÉCu£ØOH£©2³Įµķ£¬ŌņÓ¦µ÷ÕūČÜŅŗpH“óÓŚ________£»ŅŖŹ¹0.2 mol”¤L£1µÄCuSO4ČÜŅŗÖŠCu2£«³Įµķ½ĻĪŖĶźČ«£ØŹ¹Cu2£«ÅØ¶Č½µÖĮŌĄ“µÄĒ§·ÖÖ®Ņ»£©£¬ŌņÓ¦ĻņČÜŅŗĄļ¼ÓNaOHČÜŅŗ£¬Ź¹ČÜŅŗpHĪŖ________”£
£Ø3£©10 ”ꏱ¼ÓČČNaHCO3±„ŗĶČÜŅŗ£¬²āµĆøĆČÜŅŗµÄpH·¢ÉśČēĻĀ±ä»Æ£ŗ
| ĪĀ¶Č/”ę | 10 | 20 | 30 | ¼ÓČČÖó·ŠŗóĄäČ“µ½50 ”ę |
| pH | 8.3 | 8.4 | 8.5 | 8.8 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com