
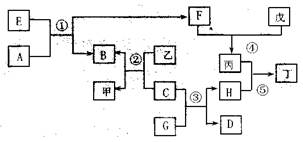
 2Cu+SO2��2�֣�
2Cu+SO2��2�֣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| Ԫ�� | �й���Ϣ |
| X | ��������������������������֮��Ϊ4 |
| Y | ����������Ӧ��ˮ����ܵ������������ȵ����������� |
| Z | �����������г�������������Ʒ�ڳ�ʪ�������ױ���ʴ���� |
| W | �ؿ��к�����ߵĽ���Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
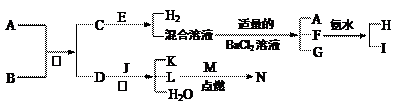 A��NΪ��ѧ��ѧ�������ʻ�����ʵ�ˮ��Һ��������֮���������ת����ϵ
A��NΪ��ѧ��ѧ�������ʻ�����ʵ�ˮ��Һ��������֮���������ת����ϵ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
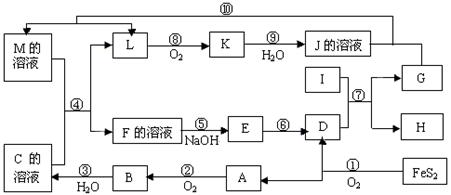
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
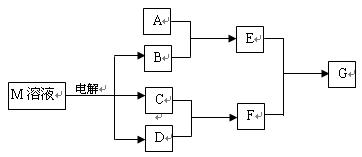

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
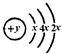 ��E�������������ǵ��Ӳ�����2�����Իش����и����⣺
��E�������������ǵ��Ӳ�����2�����Իش����и����⣺ ��
�� ������������ˮ������Һ��Ӧ�����ӷ���ʽ��
������������ˮ������Һ��Ӧ�����ӷ���ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| T | M������2�ԳɶԵ��� |
| X | �����������Ǵ�����������2�� |
| Y | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
| Z | Ԫ����������ǣ�7�� |
 ��YH
��YH ���ӵĽṹʽΪ ��
���ӵĽṹʽΪ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
|
| A��X��Y��Z��W��Ϊ�ǽ���Ԫ�� | B��X��Y��W���⻯���У�Y���⻯��е���� |
| C��Z����������Y�������ӵ��Ӳ�ṹ��ͬ | |
| D��WԪ�صļ����Ӱ뾶����ZԪ�صļ����Ӱ뾶 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com